Background
The separation of substances is nowadays performed downstream of the chemical synthesis and is usually more time and energy consuming than the synthesis itself. Thermal processes such as distillation, extraction or adsorption are dominating in this field of application Membrane separation processes are significantly less energy consuming and therefore cheaper than thermal separation processes. Due to the chemical and thermal stability of ceramic materials, it should be possible to use the membranes directly within the reactor and thus couple chemical reaction and material separation. On one hand the membrane can be used for the dosing of starting materials or on the other hand for the separation of (by-)products to shift the chemical equilibrium and thus to increase yield. It may itself be catalytically active or coated with a catalyst. This makes it possible to separate reactive intermediates and to achieve higher yields in equilibrium-limited reactions.
By coupling a catalyzed chemical reaction with a membrane that can be used for reactant-dosing or product-stripping chemical synthesis can be made much more efficient. This principle has been known for some time but has not yet been established in the industry. By using membrane reactors for chemical synthesis, new reactor and plant concepts can be developed. The combination of the catalyst with the membrane can be realized in various ways:
- Loose contact between membrane and a fixed catalyst bed or monolith
- Membrane acts as a support for the catalyst (coating)
- Catalytically active membrane
Example 1): Membrane Reactors for Chemical Synthesis: Power-to-Gas PtG / Power-to-Liquid PtL
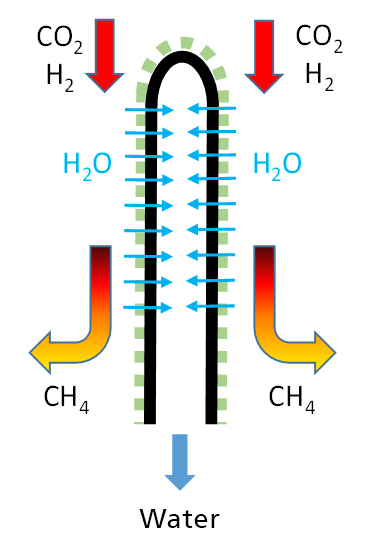
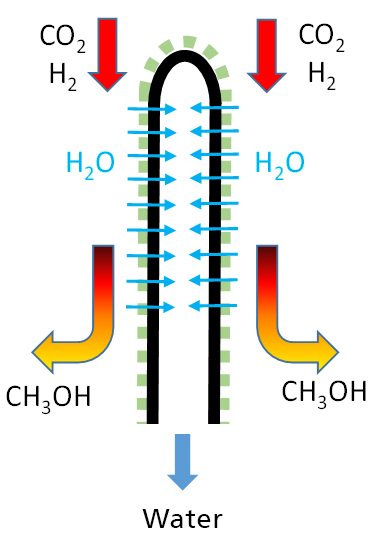
In chemical synthesis with equilibrium limitation, the membrane has the task of selectively removing a product, thereby increasing the conversion and a purified product is obtained. Examples of such reactions are the methanation (Power-to-Gas: PtG) and the methanol synthesis (Power-to-Liquid: PtL) using carbon dioxide (CO2) and hydrogen (H2) as starting materials, as both as a removable reaction product Water is created.
PtG: The Sabatier process for the production of methane (CH4) from the waste CO2 and hydrogen (H2) is currently generating great interest in the production of "artificial natural gas" for energy storage. Based on this process, hydrogen can be converted into storable methane via the "power-to-gas" (PTG) route via the intermediate hydrogen. The efficiency of the process is of crucial importance for economic efficiency. One approach to increasing efficiency is to remove the equilibrium limitation of the reaction in order to achieve higher levels of substance conversion and methane yields through appropriate measures. According to the principle of Le-Chatelier (principle of least compulsion), the position of the chemical equilibrium can be shifted within wide limits by the ratio of the reactants involved. In this case, the water is ideally removed as a reaction product to obtain highly concentrated methane. For this separation task, membranes with specific selectivity for water (steam) with retention of the other components H2, CO2 and CH4 are particularly suitable. The reaction is usefully carried out at high pressure (e.g., H2 from high pressure electrolysis). When using a membrane reactor, methane remains on the high-pressure side and, if necessary, does not have to be compressed for feeding into the natural gas network.