Electrocatalysts are serving for the acceleration of electrochemical processes, the reduction of overpotentials and therefore to improve the energy efficiency. This is especially important for the conversion of electrical into chemical energy, for example for hydrogen production from water electrolysis using excessive electric power. The theoretical decomposition voltage of water amounts to 1.23 V but at current densities relevant for technical applications substantial overpotentials are measured. Especially the 4-electron-step of the OER (Oxygen Evolution Reaction) is critical for overall efficiency. The losses caused by overpotentials are converted into heat not usable for the process. Accordingly, the efficiency of the water electrolysis is directly restricted by the overpotentials at the electrodes.
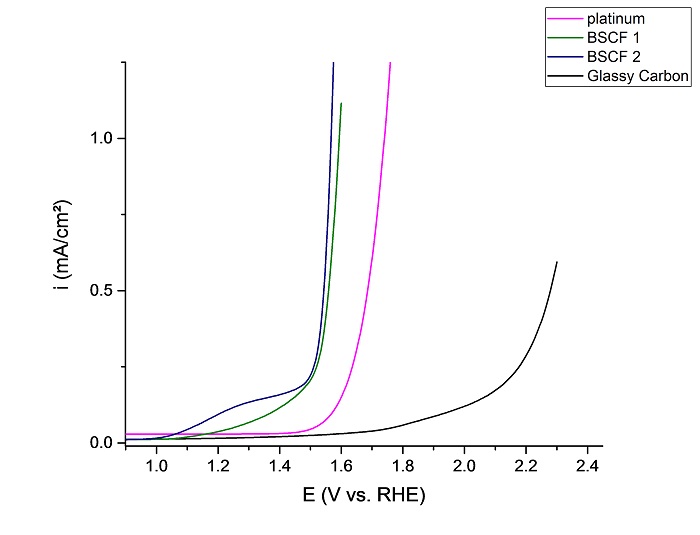
Electrocatalysts are able to reduce the overpotentials substantially and to increase the efficiency of the electrolyzer. For water electrolysis, OER at the anode offers the biggest potential for increasing the efficiency due to the fact that the anode overpotential is typically twice as high as on the cathode side. Furthermore, low-cost oxidic electrocatalysts can be used instead of expensive precious metals as the accompanying diagram proves. For the use in real electrolyzers the development of suitable coating techniques as well as improving the conductivity of the composite anodes is necessary.
Services offered
- Characterization of electrocatalysts
- Determination of electrocatalytic properties
- Adaption of material properties (particle morphology, conductivity)
- Application as coating for variable parts and devices
Technical equipment
- Test rig for electrocatalytic characterization (e.g. rotating disc electrode)
- Galvanostat for stability test under galvanostatic conditions
- Milling units for morphologic adjustment
- Coating systems and processes for application on surfaces, form bodies