
Electrocatalysts for improving the efficiency of alkaline water electrolysis
Current research


The extensive use of renewable energy leads to fluctuating feed-in of energy to the grid, giving rise to a need for highly efficient storage of excess energy. Conversion into chemical energy is especially suitable for long-term storage. Water electrolysis is a promising process in the context of “power-togas” strategies.
The electrolysis process efficiency is proportional to the cell voltage and directly influences the overall storage process efficiency. All real electrolysis units suffer from overpotential in both the anode and the cathode reaction, with the fourelectron anode reaction having the highest overpotential. Electrocatalysts can significantly lower the required cell voltage. Thus, use of inexpensive electrocatalysts offers great potential for increasing efficiency.
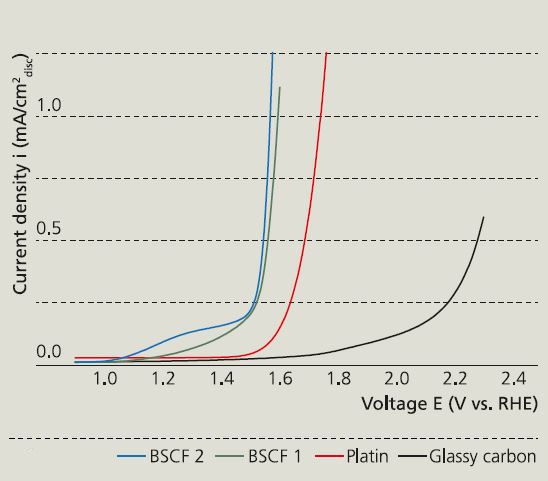
Figure 1 shows the decomposition voltage in alkaline water electrolysis without a catalyst (glassy carbon = catalyst support) as well as for platinum and Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF)-coated electrodes. As the voltage was being raised, a substantial current flux already occurred below the theoretical decomposition voltage of 1.23 V. This was due to oxidation of Fe/Co in the BSCF catalyst. With respect to the oxygen evolution reaction, the required voltage was observed to approach the expensive platinum catalyst level (shift to the left).
During use under alkaline water electrolysis process conditions, the cell voltage reduction amounted to ca. 100 mV by using an electrode as shown in Figure 2 at a current density of 1500 A/m², corresponding to an efficiency increase of 4 %. Through adaptation of the coating process, a cell voltage reduction of 300 mV at a current density of 5000 A/m² was achieved in the electrolysis test rig using an electr ode. This yielded an efficiency increase of 12 % over that of the standard setup without electrocatalysts. Furthermore, the applied coating was chemically and electrochemically stable.
Services offered
- Development of electrocatalysts
- Electrocatalytic activity measurements
- Coating of electrodes
Acknowledgments
The German Federal Ministry of Education and Research BMBF and Project Management Jülich are gratefully acknowledged for their financial support. All partners involved in the joint project “Katalytische Mischmetalloxide” of the innovative regional growth core “Partikeldesign Thüringen” (grant no. 03WKCN02C) are also thanked.