Electrolysis

High-temperature electrolysis with SOE stacks
High-temperature electrolysis has many advantages. In contrast with established alkaline or PEM electrolyzers, it does not require noble metal components. Integrating high-temperature electrolysis in processes where a lot of waste heat is produced – as in steel production – efficiency can be quite significantly increased compared to competing technologies. Additionally, high-temperature electrolysis enables the direct production of syngas.
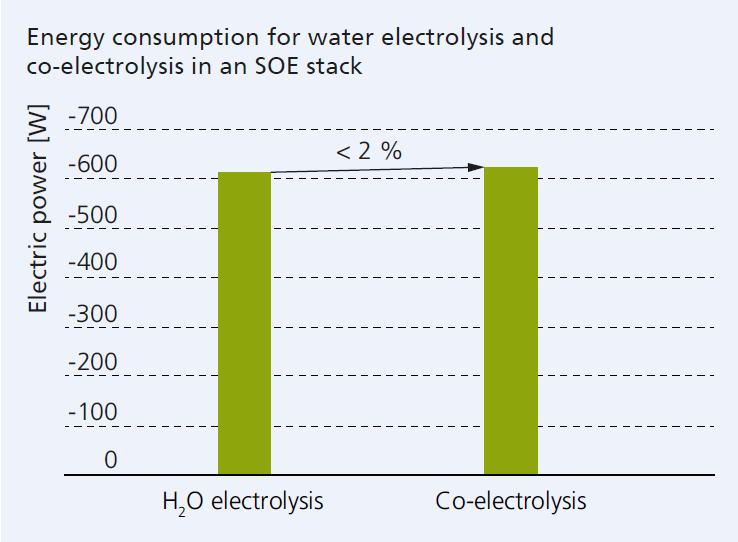
The conversion processes in high-temperature electrolysis, also called solid oxide electrolysis (SOE), take place at temperatures of more than 750 °C. The key components are SOE stacks, which are able to work in electrolysis mode or in fuel cell mode. Applying a voltage across an oxygen ion-conducting electrolyte separates water vapor into hydrogen and oxygen. Furthermore, the so-called co-electrolysis mode makes it possible to use unavoidable, climate-damaging CO2 for the production of climate-neutral products. In this process, water and CO2 are split into oxygen, hydrogen and carbon monoxide. At its end, electricity allows for the production of syngas which can be stored or converted into higher-value products (see ‘Hydrogen utilization‘).
Fraunhofer IKTS has been working with SOE for more than 25 years and possesses extensive know-how across the complete value chain, from material to system, including analyses of economic viability. In recent years, developments at the institute have culminated in the incorporation of very successful new companies. Fraunhofer IKTS produces stacks and modules for integration in electrolysis plants on a pilot scale and optimizes them with regard to their long-term stability and performance (e.g. by increasing the current density up to 0.75 A/cm2). The main focus is now on developing and testing industrial-scale automated stack production and modularization concepts for higher performance classes.
High-temperature electrolysis with proton-conducting electrolysis cells
In addition to oxygen-conducting electrolytes as used in SOE stacks, Fraunhofer IKTS also develops proton-conducting materials for electrolyzers used at temperatures between 550 to 600 °C. They provide hydrogen in a highly pure, water-free form, without any further treatment steps. If this process is combined with exothermal synthesis processes, the reaction heat can be used directly to supply the electrolysis with energy. This significantly increases the efficiency of the chemical conversion processes.
The main parameters for the use of proton conductors are proton conductivity, chemical stability and the polarization resistance between electrode and electrolyte. For this purpose, Fraunhofer IKTS develops electrolyte and electrode materials and converts them into planar stacks, in analogy with SOE technology.
Alkaline large-scale electrolysis
Alkaline electrolysis (AEL) is an established industrial process for the production of hydrogen and oxygen. It uses an OH–-conducting liquid electrolyte and is operated at a temperature of approx. 80 °C. Thanks to its high maturity and low specific investment cost compared with other electrolysis technologies, it is currently the most widely applied process worldwide. However, alkaline electrolysis has not yet truly penetrated the market and automated production processes have not been established to date. This is why IKTS is optimizing stack and system components with regard to their energy density (current energy densities below 0.5 A/cm2) and ecological as well as design aspects (reducing the use of noble metal catalysts, increasing the active electrode area). Through long years of experience in SOE and MCFC stack construction, it is possible to make major steps toward improving ecological and economic parameters.
Membrane electrolysis
Using low-temperature membrane electrolysis, it is possible to extract hydrogen efficiently from industrial and mining waters. Fraunhofer IKTS has developed its RODODAN® process for this purpose. In this process, the electrochemical treatment of sulfuric and sulfate-rich waters also produces, in addition to sulfate and iron, hydrogen as a usable byproduct. This process takes place in a membrane electrolysis cell whose electrode spaces are separated from each other by an anion exchange membrane. While a current is flowing, hydrogen ions are discharged at the cathode and hydrogen is removed as gas. Fraunhofer IKTS uses the findings obtained from the use of anion exchange membranes in this process for the further development of modern alkaline membrane electrolyzers (AEMEL).