

Electrochemical energy storage systems and converters based on alkali metals, such as sodium (Na/S, Na/NiCl2, AMTEC), are highly efficient and challenging components, which operate at high temperatures. Ceramic alkali-ion conductors, serving as solid electrolyte and separator, are main component of these batteries. Doped ß‑aluminates, whose ceramic synthesis is challenging, are an important representative of these materials. Fraunhofer IKTS is able to produce ß‑aluminates in the kg-scale based on different synthesis routes.
The electrochemical characterization of this material in specific high-temperature cells is conducted on test bodies, such as rods, tablets or cups. The ionic conductivity, the contact resistance between ceramics and anode or cathode, as well as the degradation behavior are determined and an optimal raw material basis with adjusted process route is thus developed.
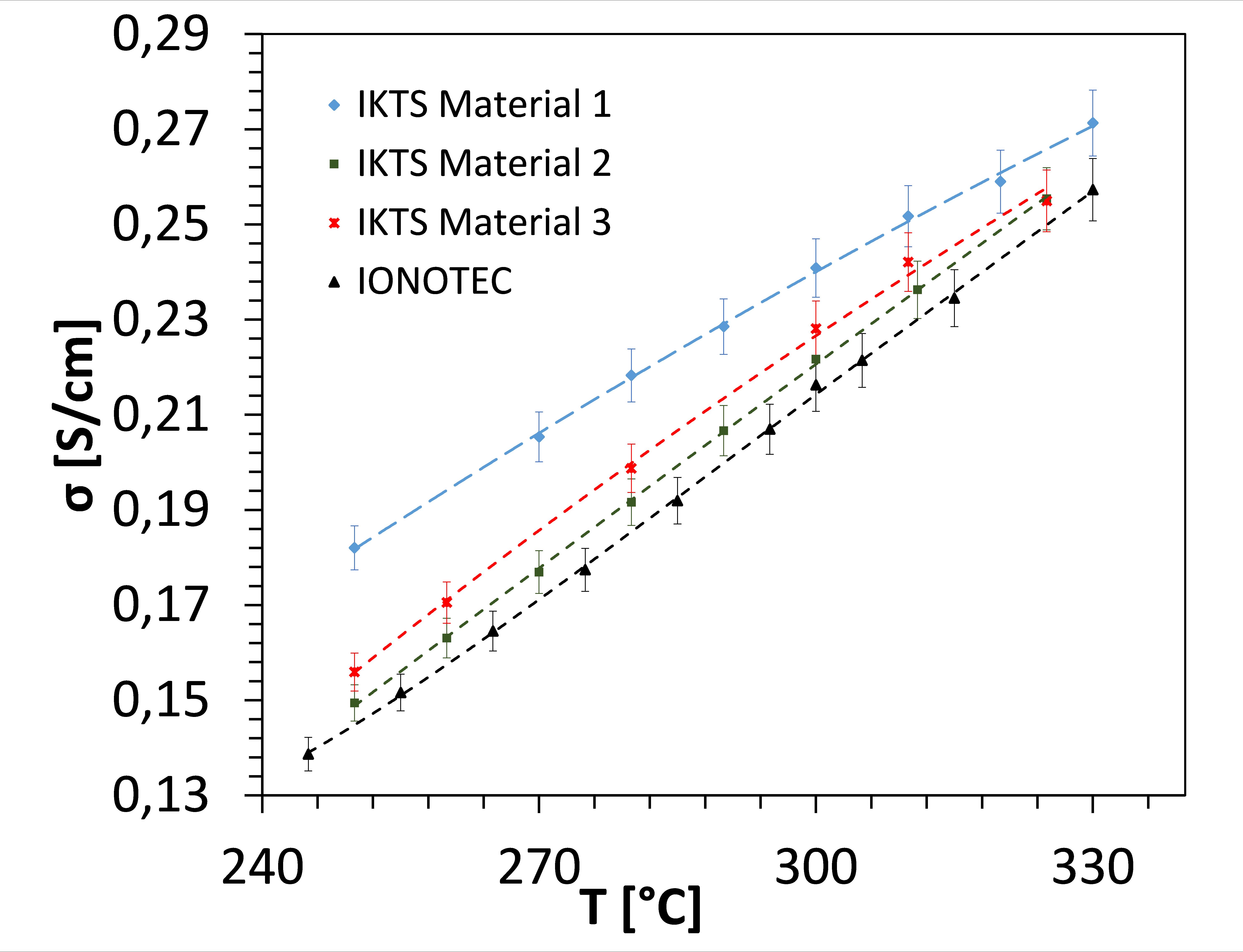
Electrolyte tubes with a length of up to 300 mm can be realized by pressing. The physical and electrochemical properties of the components are among the global leaders. Ionic conductivities up to 0.241 S/cm (300 °C) and high densities can be proven. Alternative manufacturing processes, such as tape casting or extrusion, are being developed in order to establish highly efficient, cost-efficient shaping for mass production.
Services offered
- Development and synthesis of alkali-ion conducting ceramics on the basis of ß‑aluminate in the laboratory and pilot-plant scale
- Production of test bodies and electrolytes (tablets, cups, tubes)
- Set-up of high-temperature cells for characterization
- Tests in full and half cells (Na/S, NaNiCl2): charge-discharge behavior, degradation behavior
- Determination of Na-ion conductivity in ceramic solid electrolytes in the temperature range up to 400 °C
Technical equipment
- High-temperature test cells for the electrochemical characterization of the alkali-ion conducting ceramics
- Impedance-capable potentiometer up to 80 A
- Glove boxes for water- and oxygen-free operations
- Equipment for ß‑aluminate synthesis from the gram to the kilogram scale