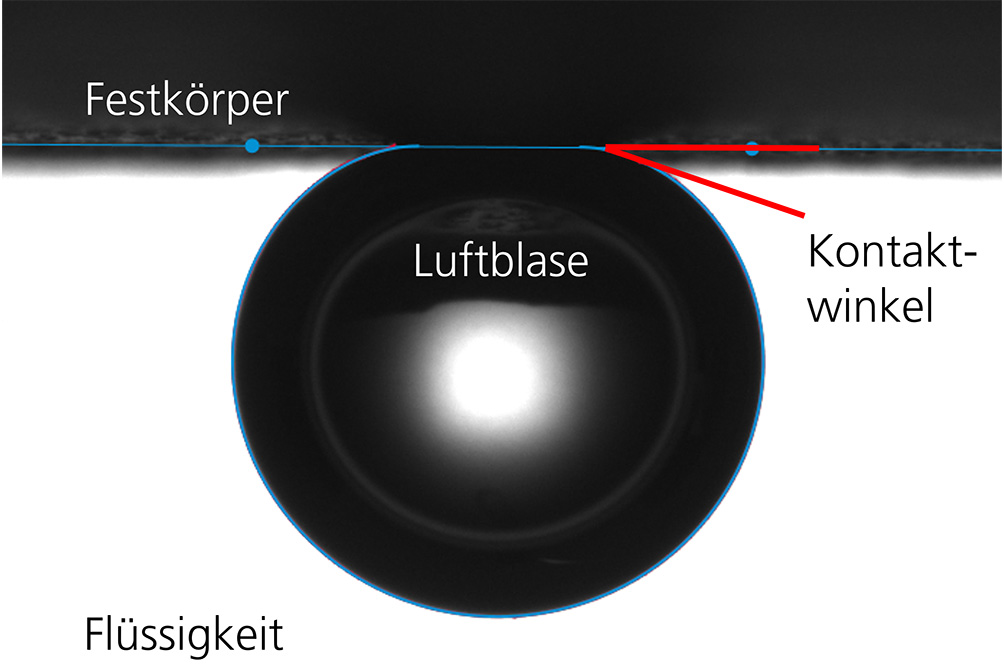
The captive bubble technique uses a special setup that allows contact angle measurement even when the conventional "lying drop" method (sessile drop technique) does not work.
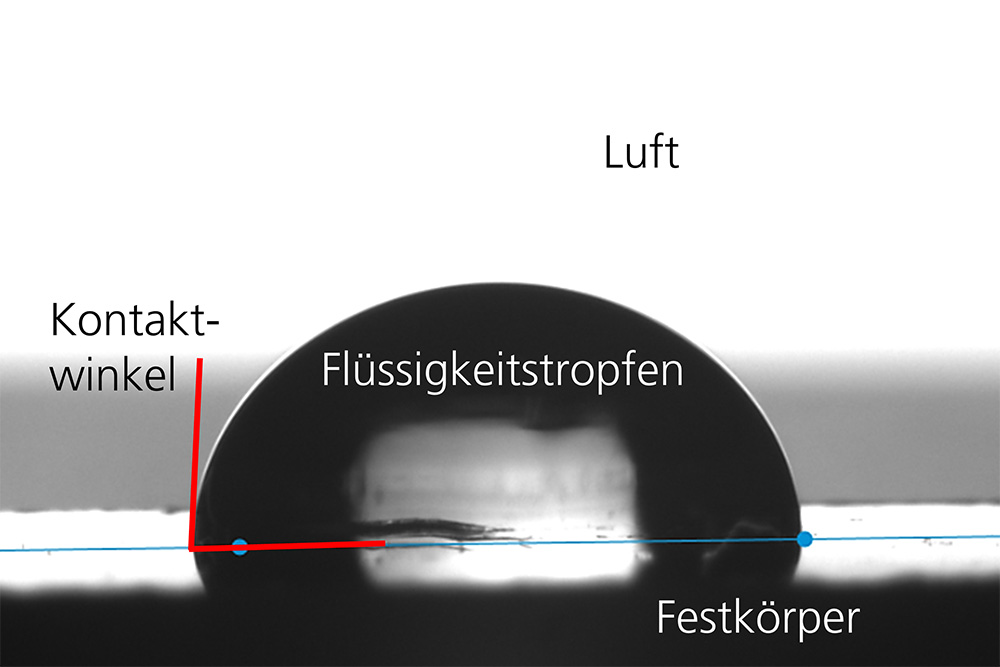
The contact angle formed by a drop of liquid on a solid surface provides information about the wettability of the surface with this liquid. Small contact angles (< 90°) correspond to high wettability and large contact angles (> 90°) to poor wettability. The contact angle is influenced by both the surface tension of the liquid and the chemical and physical properties of the solid. Contact angle values can be used to determine its surface free energy and its disperse and polar fractions.
Typically, the contact angle is determined using the horizontal drop method, in which drops are placed on the solid surface from above. However, this method is not suitable for surfaces with high surface free energy on which liquids spread. For this purpose, Fraunhofer IKTS uses the captive bubble technique. The solid surface is completely immersed in the liquid and an air bubble is dispensed below the surface. "Trapped" at the surface, the contact angle is formed at the three-phase point between solid, liquid and air bubble.
This technique can be particularly advantageous for strongly hydrophilic surfaces, because here hydration under water is complete and thus defined. Measuring the contact angle under water is also useful if the surface to be tested is intended for use in water. Then we can study processes at the interface, for example the fouling of a solid surface with a biofilm.