Synthesis gas (CO + H2) takes a key position in many processes of substance and energy conversion. It is generated by partial oxidation or reforming of hydrocarbons and is the base for Fischer Tropsch synthesis of synthetic fuels from natural gas as well as "green" renewable fuels from biomass. A requirement for such a material utilization is a synthesis gas free of nitrogen, as it results from the partial oxidation using pure oxygen (O2). In contrast to that, a partial oxidation with air delivers a N2 containing synthesis gas which could preferably used in gas engines and fuel cells. Also in that case, electrical efficiency can be significantly enhanced by exclusion of N2.
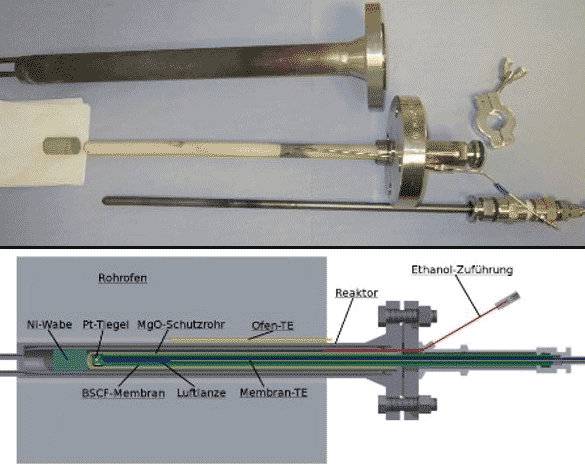
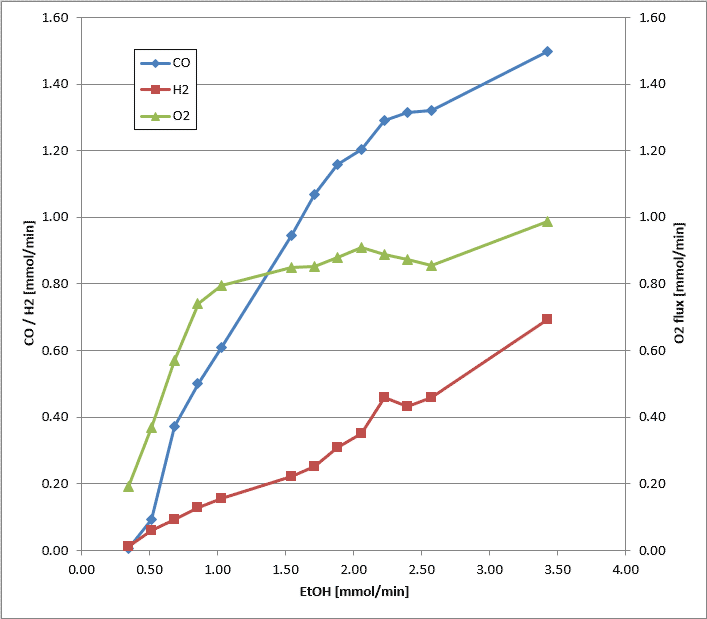
In general, the benefit of a N2-free synthesis gas is in contrast to the effort for O2 provision. Its production directly in the membrane reactor has the important advantage that the driving force for the separation of O2 is provided by the reaction itself and that no additional energy is required. However, the majority of the MIEC materials (Mixed Ionic Electronic Conductor) are not sufficient long-term stable in the presence of CO and H2 under operation conditions, but materials more stable have a lower O2 permeation. Therefore, a constructive approach was pursued according to figure 1 characterized by the use of a porous ceramic protecting the MIEC membrane against a direct attack by CO and H2. Figure 2 shows the influence of the ethanol throughput on the composition of the product gas. The long term stability is still insufficient for a continuous industrial process, but allows already a reasonable use for mobile fuel cells.