
Tubular membrane-electrode assembly for solid-state ammonia synthesis
Current research

The desired conversion to a low-emission economy requires the development of innovative CO2-free processes for energy conversion and the adaptation of existing industrial processes. In addition to methanol, ammonia plays a major role as a completely carbon-free energy source for that transition process. Compared with hydrogen, ammonia has a significantly higher energy density and can be stored and transported more efficiently and cost-effectively as a liquid.
Ammonia is already one of the most important and most-produced basic chemicals worldwide. However, the current state of the art – ammonia synthesis using the Haber-Bosch process – is one of the largest sources of CO2 emissions and is responsible for up to three percent of global fossil fuel consumption. The development of alternative synthesis routes for green ammonia will therefore become a major challenge of the next decade.
Solid-state ammonia synthesis
A possible solution is being pursued as part of the joint project CAMPFIRE 04 with partners from the Leibniz Institute for Plasma Science and Technology (INP), the Hydrogen and Fuel Cell Center (ZBT) and the Leibniz Institute for Catalysis (LIKAT). The investigations focus on decentralized solid-state ammonia synthesis (SSAS) based on thin-layer-based membraneelectrode assemblies, using renewable energies (power-to-ammonia). The SSAS is an electrochemical ammonia synthesis route, in which a direct conversion of atmospheric nitrogen and water evolves in a membrane reactor. On the anode side, water is decomposed into oxygen and protons. The protons are transported to the cathode via a proton-conducting membrane to the cathode, where they react directly with atmospheric nitrogen.
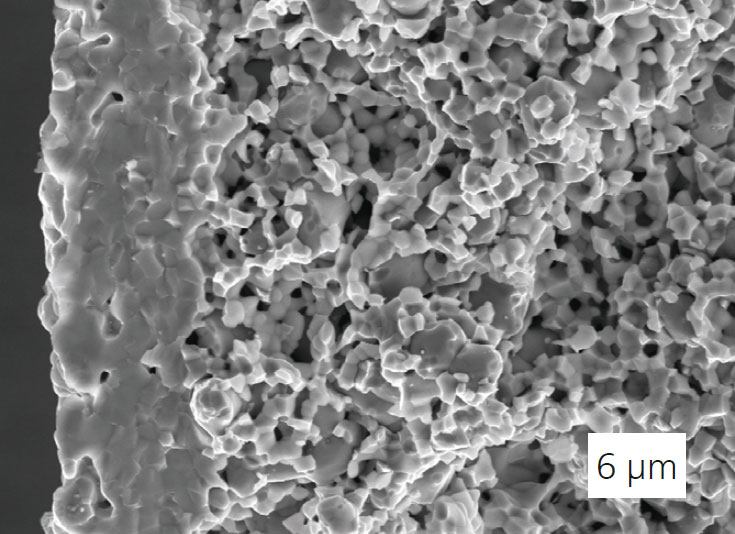
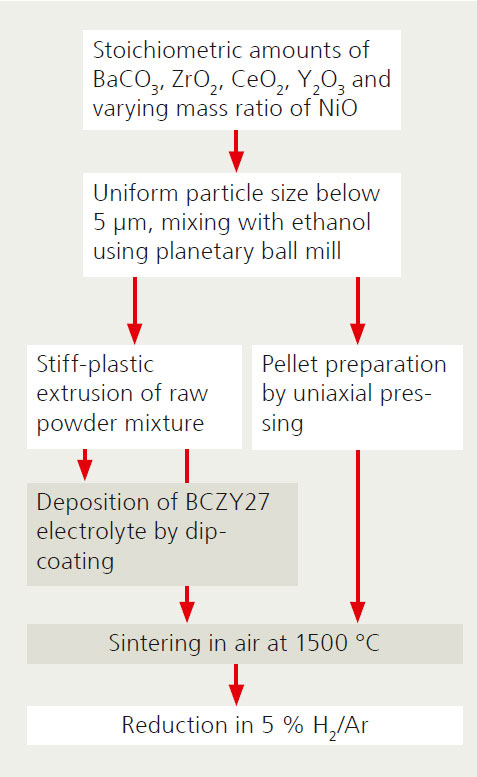
The heart of the membrane reactor, the tubular porous composite support (metal/ceramic) for the hydrogen-conducting electrolyte was developed at Fraunhofer IKTS. The tubular design is crucial for the pressure stability of the membrane reactor. The open-pored support also enables rapid gas-phase transport thanks to its fine pore structure.
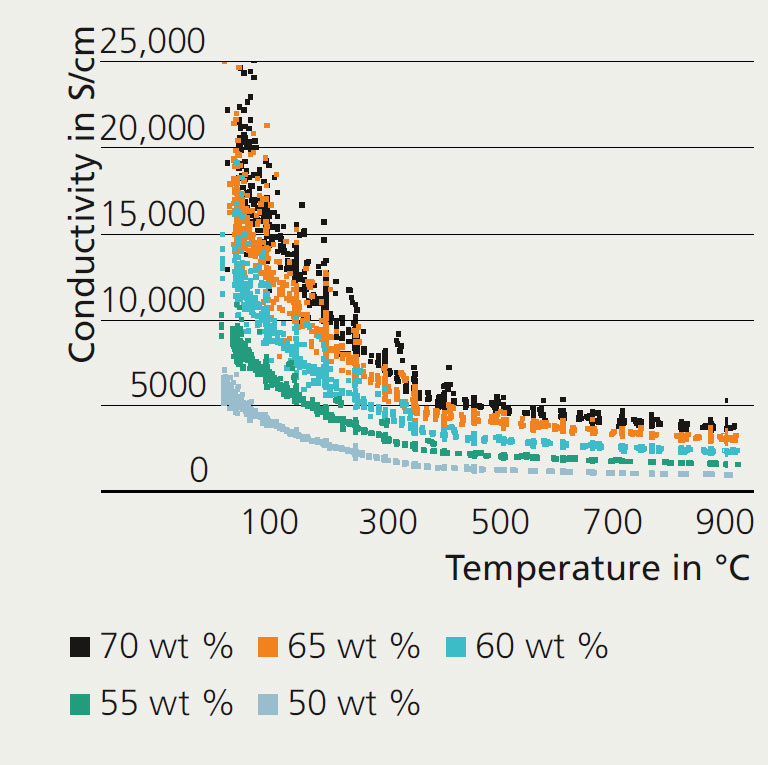
The optimized percolation structure consisting of metallic nickel and ceramic proton conductor (BaCe0.2Zr0.7Y0.1O3) is the prerequisite for the high electric conductivity of the membrane support.
Fraunhofer IKTS developed the recipe for the stiff-plastic extrusion of raw powder of the support composition and adapted it to the requirements of the final component strength. Values of 86 MPa were measured for tubes with an outer diameter of 10 mm, which represents a satisfactory pressure stability.
Services offered
- Customer-specific support and membrane development for the SSAS
- Plastification of ceramic starting powders and extrusion of tubes and honeycombs of different geometries
Supported by

