The Fischer-Tropsch synthesis represents a central process stage of numerous power-to-X concepts for the sustainable production of high-quality chemical products from CO2 and H2O using renewable energy. Essential features in the product synthesis on iron-based catalysts, i.e. for the production of higher alcohols, are the presence of numerous catalytically active phases, a large reaction network and a product mixture of hydrocarbons of different substance groups and chain lengths. Due to this complexity, the underlying mechanism of the reaction is not yet sufficiently understood, hindering process optimization according to ecological and economic criteria. Utilization of in-situ measurement techniques provides promising new insights into previously inaccessible information.
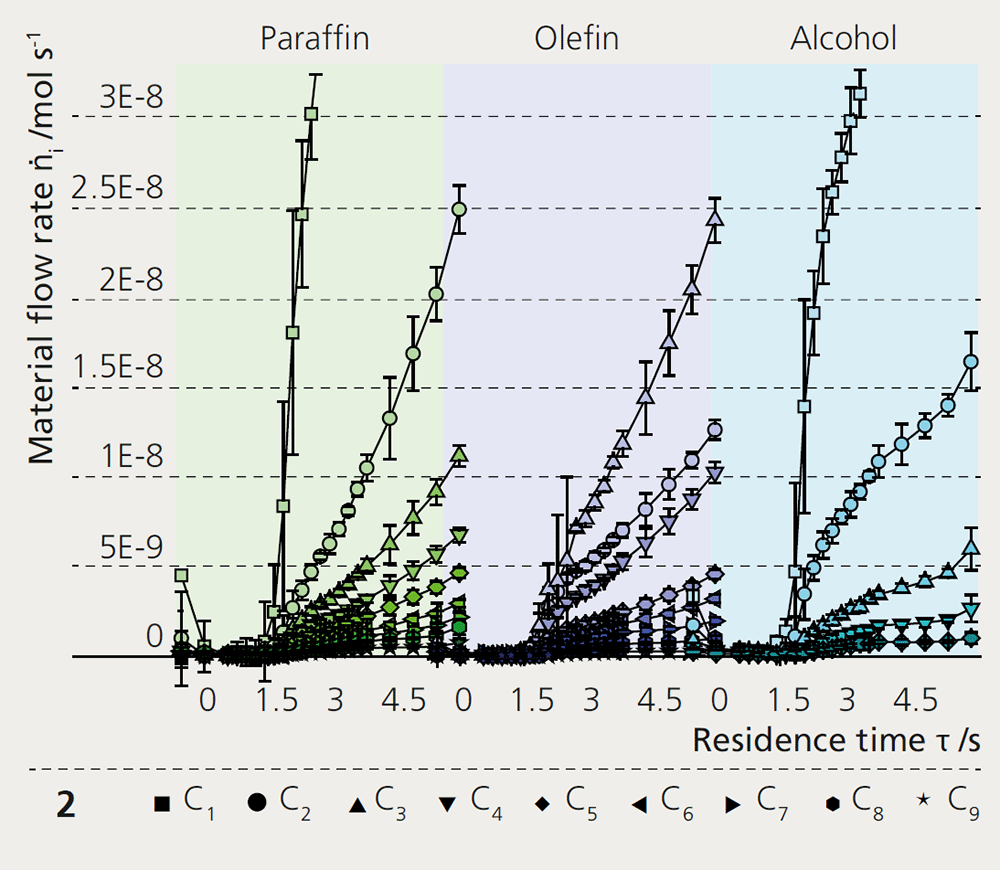
With a novel reactor concept called ‘profile reactor’, it is now possible to look inside tubular reactors and follow directly the main, secondary and parallel reactions based on product distribution. A capillary, which is centrally located in the catalyst bed and can be moved relative to it, is used for sampling. The composition of the gas phase thus obtained is analyzed by gas chromatography. This yields spatially resolved reaction profiles, which allow conclusions to be drawn about the character of the chain growth, kinetic parameters such as the reaction rate, re-adsorption and on secondary reactions of by-products.
The variation of catalyst composition and operating conditions gives access to structure-activity relationships inside the catalyst. The analyses were able to show a strong correlation between the extent of ongoing secondary reactions and the operating conditions. In particular, oxygenates such as higher alcohols, which are promising products of the Fischer-Tropsch synthesis on iron catalysts, have a strong tendency, due to their chemical structure, to be converted into undesired secondary products in side reactions. Therefore, an understanding of the reaction mechanism is particularly relevant for optimizing the yield of higher alcohols. Specifically, it could be shown that by targeting the adjustment of temperature, pressure and catalyst loading, these unwanted reactions can be minimized. In combination with a tailored catalyst composition applying promoting elements like potassium, copper or molybdenum, it becomes possible to optimize selectivity towards higher alcohols.
The established methodology can also be used for the investigation of reaction mechanisms of other complex reactions.