
Study on the kinetics of flexibly operated iron ore direct reduction
Current research



The steel industry causes approx. 7 % of global anthropogenic CO2 emissions and is therefore currently one of the biggest emitters of CO2. The direct reduction process, as an alternative route to conventional steel production, offers enormous potential for CO2 reduction, especially as it ideally facilitates the changeover to sustainable power generation and can be implemented relatively shortterm.
In direct reduction, pelletized iron ores are converted into sponge iron using gaseous reducing agents, instead of coal and coke as in the conventional blast furnace process. Natural gas can be used but regeneratively produced reducing gases, such as hydrogen or syngas, are also suitable. The flexible operation of direct reduction plants with dynamic changes in the raw material base can offer enormous advantages compared with stationary operation. It is conceivable to control production for grid-connected services while making optimal use of volatile renewable energy sources, and to increase economic efficiency and resilience to changes in the energy market.
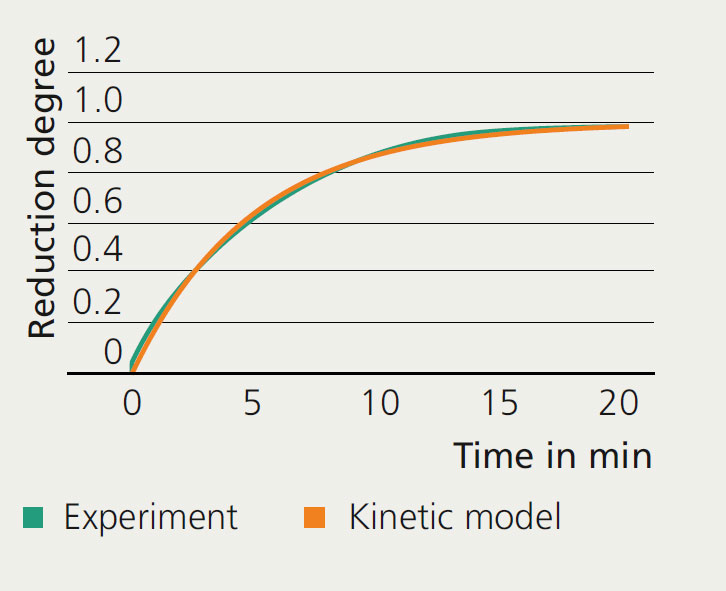
To evaluate the potential of the process, knowledge of the reaction kinetics of flexible direct reduction is essential. As part of the BMBF-funded project ”BeWiSe“, a direct reduction plant was implemented on a laboratory scale, which makes it possible to investigate the reaction kinetics on individual iron ore pellets at different process gas compositions and reaction conditions up to 1000 °C and 8 bar. In addition, the system is equipped with an automated conveyor unit, which allows the reactor to be filled and emptied while it is still hot, thus ensuring that experiments are carried out in a time-efficient manner.
Based on the experimental work, a comprehensive kinetic model is currently being developed in which the influence of each significant parameter is considered. The necessary quantification of the reaction rate constant is very complex due to the many different mechanisms involved. For the heterogeneous gas-solid reaction, the kinetic regime can be determined through the pore diffusion of the gases within the pellet, solid-state diffusion of Fe2+ and O2- ions or by nucleation and growth during the phase transition. In the presence of carbon-containing components in the process gas, the formation of carbidic phases, the deposition of pure carbon and the water gas shift reaction also occur.
The model developed will be used to simulate the transition conditions that occur in the flexible operation of the direct reduction process in order to be able to predict energy demand, the degree of metallization and other process parameters.
In addition to the direct reduction of iron ore, the plant, which is unique at Fraunhofer IKTS, can be used for a variety of other applications in the field of pressure-loaded high-temperature processes.
Sponsored by
