
High-temperature electrolysis for the production of green ammonia
Current research

Ammonia is considered a key chemical in a future sustainable energy and raw materials system. It is the most important basic chemical for fertilizer production and thus essential for feeding the growing world population. In the energy sector, ammonia is considered as a future carbon-free energy carrier with high energy density. The starting materials for the synthesis of this important chemical are nitrogen (N2) and hydrogen (H2). In the EU-project ARENHA as well as in the BMBF-funded project GreatSOC, Fraunhofer IKTS investigates ways to convert renewable energies into ammonia in the most efficient way.
High-temperature electrolysis
The supply of green (sustainably produced) hydrogen can only be achieved on a large scale via electrolysis. High-temperature electrolysis (SOE) operated at 700–850 °C has an outstanding role here, as it can produce hydrogen in a particularly energy-efficient manner through various heat integration options (electrical energy requirement: 3.2–3.5 kWh/Nm³ H2). Cells, stacks and stack modules for this technology are being continuously developed and tested at Fraunhofer IKTS.
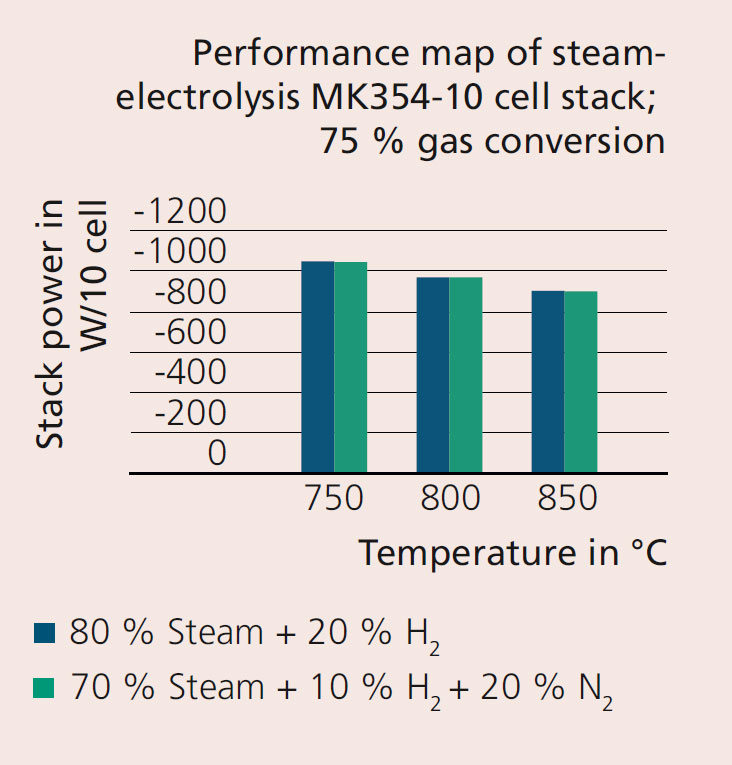
For ammonia synthesis, a reactant gas mixture of nitrogen and hydrogen in a ratio of 1:3 is required. The results presented in Figure 2 show that nitrogen admixture in electrolysis has no influence on the power required to supply hydrogen. This forms an important basis for innovative concepts for the supply of synthesis gas for ammonia synthesis via high-temperature electrolysis.
Sustainable concepts for the production of ammonia mostly focus on the production of the synthesis gas (N2, H2) for the downstream Haber-Bosch reactor, in which the actual conversion to ammonia takes place. When using low-temperature electrolyzers, air separation is required as an additional process step for the recovery of nitrogen from air. High-temperature electrolysis, on the other hand, allows the synthesis gas to be produced and supplied directly. For this, part of the hydrogen produced can be reacted with air to supply nitrogen. The resulting water can be converted back into hydrogen in the electrolyzer.
An additional advantage is that high-temperature electrolysis can use the waste heat from exothermic process steps, such as ammonia synthesis, to increase energy efficiency. This enables up to 10 % lower energy requirements for ammonia production compared to low-temperature electrolysis-based process concepts.
Unique core competencies of Fraunhofer IKTS, such as the simulation of individual process steps/reactors and their coupling with experimentally obtained operating data, allow an optimized process design up to demonstration.
We thank the European Union for funding the ARENHA project under the Horizon 2020 research and innovation program (funding no. 862482) and the German Federal Ministry of Education and Research BMBF for funding the GreatSOC project (funding no. 01DR22005A).
Sponsored by


