
Cold sintering – New ways to manufacture and integrate functional ceramics
Current research

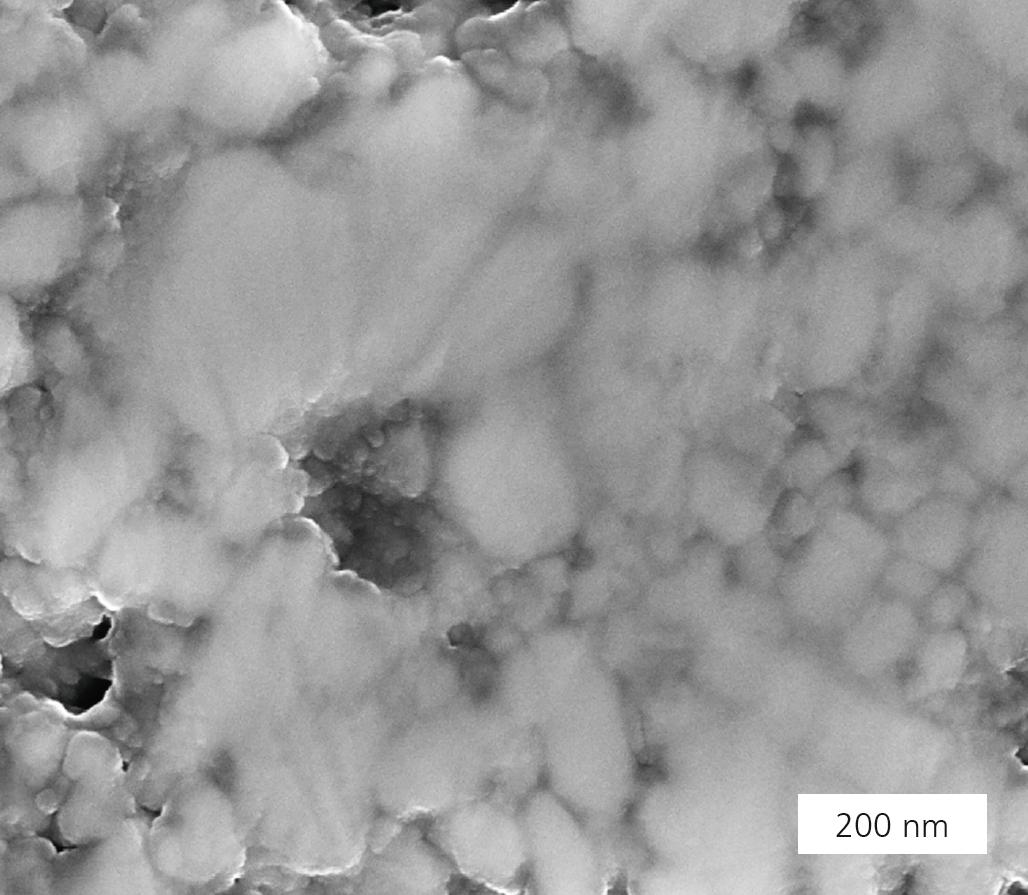
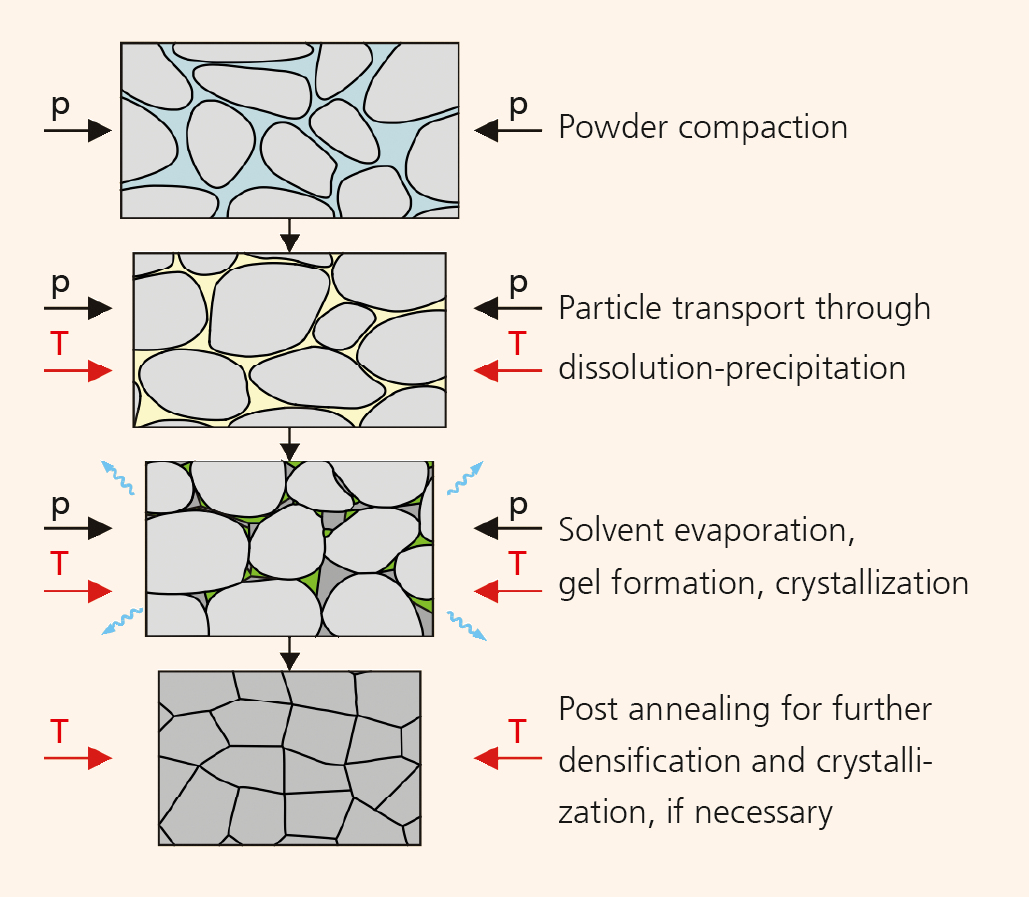
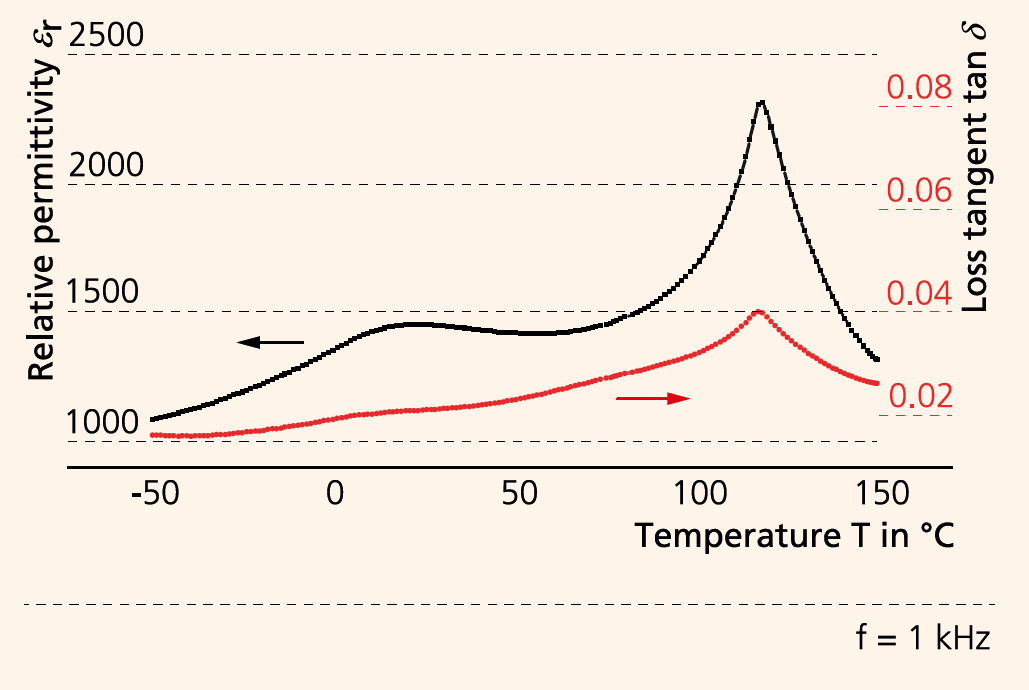
Cold sintering promises densification of ceramic materials at temperatures below 300 °C. The process uses transient solvents which aid densification through a mediated dissolution-precipitation process. The congruent solubility of a ceramic material in a suitable solvent (e.g. aqueous solution) is a prerequisite for this. With the aid of the liquid phase, the solid particles are rearranged under moderate temperatures and high pressures. The diffusive mass transport is followed by the precipitation process, where amorphous phases form at the grain boundaries. After the solvent has evaporated, these phases can either remain amorphous or recrystallize, depending on the material properties (Figure 2). This paves the way for completely new material combinations, such as the monolithic integration of different ceramic materials (e.g. oxides and nitrides), the combination of different microstructures (e.g. nano and micro), and the preparation of ceramic-polymer composites in a single step. All this requires that suitable pressure and temperature environments are determined. At Fraunhofer IKTS, research into different ceramic materials, such as LiFePO4, BaTiO3, (K,Na)NbO3, ZrO2 and TiO2, has shown that ceramic materials vary in their suitability for cold sintering. Process parameters were determined which allow for the manufacturing of monolithic ceramic samples. LiFePO4, for example, can be cold-sintered to 89 % relative density at 220 °C/ 150 bar (Figure 1). In contrast, BaTiO3 requires additional thermal annealing. The phase transition temperatures determined for BaTiO3 show a good agreement with samples manufactured under conventional thermal sintering conditions. However, standard material parameters have not yet been reached, which makes further research into cold sintering necessary. The cold sintering process is therefore especially suited for materials with low lattice energies, reactive surface states, ionic bonding and reduced hydrolytic resistance.