

Increasing efficiency by in-line dewatering at supercritical conditions
Many industrial applications require new morphologies, for example for different polymers, temperature-sensitive biopolymers, or nano-composites. One way to create these materials is to dewater them under supercritical conditions, a method used in the Pressurized Gas Extended (PGX) Technology. This application uses a combination of carbon dioxide and ethanol at approximately 100 bar. This so-called supercritical mixture then absorbs water during a spray drying process. In order for PGX to be efficient, the mixture of ethanol and carbon dioxide must constantly be recycled, i.e. relieved of water. Conventionally, the mixture is dewatered after it is expanded to normal pressure. A disadvantage of this technology is that the mixture must then be re-compressed. An in-line application capable of dewatering at supercritical conditions would greatly improve the efficiency of the entire process.
Membranes hold up under supercritical conditions
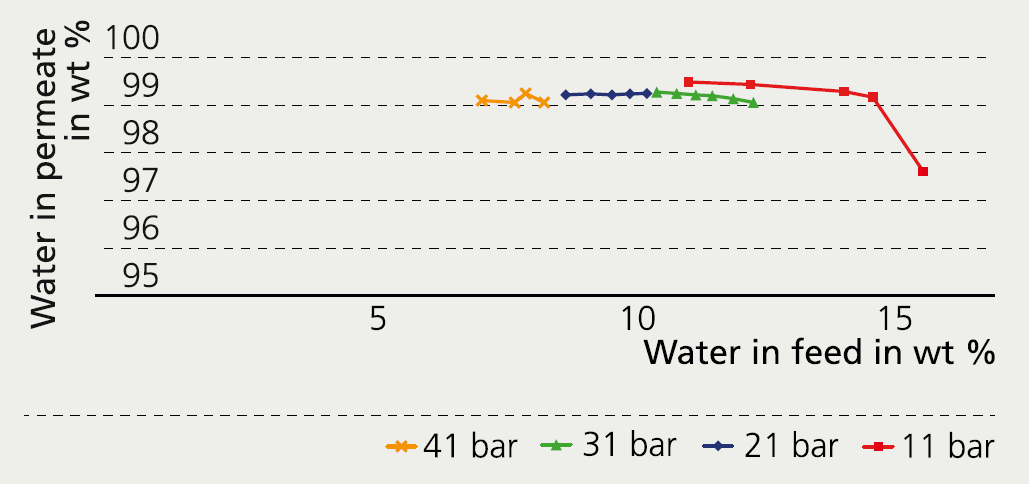
Fraunhofer IKTS has developed hydrophilic membranes which can be used for dewatering solvent-water mixtures by pervaporation or vapor permeation. However, dewatering at critical conditions places high demands on the chemical and mechanical stability of the membranes. In a German-Canadian research project, zeolite and carbon membranes were evaluated, further developed, and tested in the supercritical fluid application. Pore size and chemical stability of the active layer and the mechanical resistance of the extruded membrane layer had to be taken into account for this. In extensive lab trials, carbon membranes coated on membrane supports of increased mechanical stability demonstrated convincing separation properties and high selectivity at increased pressures. Even at pressures above 40 bar, almost pure water was extracted from a water/ ethanol/carbon dioxide mixture. The membranes are currently being evaluated by the Canadian project partner CEAPRO in separation tests at supercritical conditions. Prevailing separation characteristics are very positive, making a future implementation in the industrial process most likely.

