The combination of bioceramic materials and biophysical system developments will create additional synergies between the ceramic and sensory-actuator-micro systems, enabling the production of innovative products with significant added value in diagnostic and therapeutic medical equipment.
Biophysical sensors
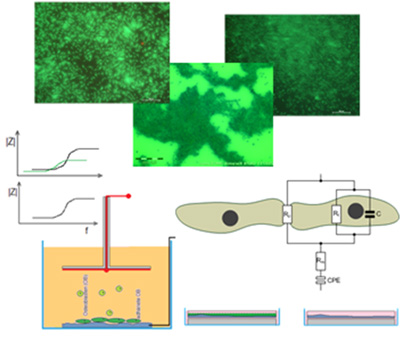
- Bio electrical and topographic measurement systems
- Monitoring of cell-/tissue- and microbial growth on surfaces bio functional / implants -> Bioceramics
- Development of in vitro systems analysis
Biophysical actuators
- Stimulation and suppression of cell, tissue and microbial growth on bio functional ceramic surfaces and implants
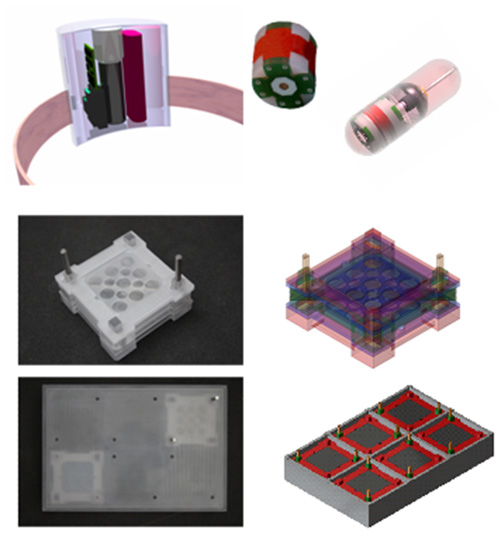
- Scalable power transmission systems ex vivo and in vivo (in situ - applications)
- Stimulation & suppression actuator systems -> Energy Systems
- Development of in vitro and in vivo treatment systems
Theranostic sensor-actuator systems
- Research on the combination of diagnostic and therapeutic capabilities in integrated applications
- Analysis and control software development
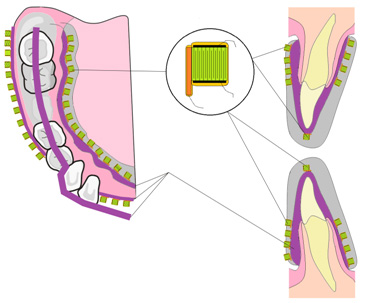
Bio ceramic surface and mold design
- Bio morphologic designed surfaces
- Bio mimetic designed crowns, bridges and implant molds
- Integration of bio interfaces
Competence and Facilities
- Completion of micro systems to solve measurement problems in biological in vitro models
- Development of cell-/tissue analytic module of biocompatible materials, control surfaces, plastic and ceramics as well as the integration of functional electrical components. This covers the complete process chain from computer-aided design and simulation of the transfer in the production to a variety of manufacturing technologies that can enable a highly efficient design and manufacture of biocompatible or bio inert composite systems made of ceramics, plastics and metals.
- Technical equipment and expertise in the field of micro systems engineering, scientific instruments and the measurement of ceramic samples with respect to the characterization of conductivity behavior of dielectric materials, magnetic materials and semiconductors. The institution's internal laboratory is accredited for quality and reliability of measurements in this area according to DIN EN ISO 17025.
- Support medical device development, including QMS (EN ISO 13485, § 30 MPG), Risk Management (ISO 14971) and clinical evaluation (after MEDDEV)
- Targeted development of biomaterials with biological systems adapted for surface structure and composition in cooperation with other groups of IKTS