
Plasmaelectrolytic oxidation of magnesium
Current research

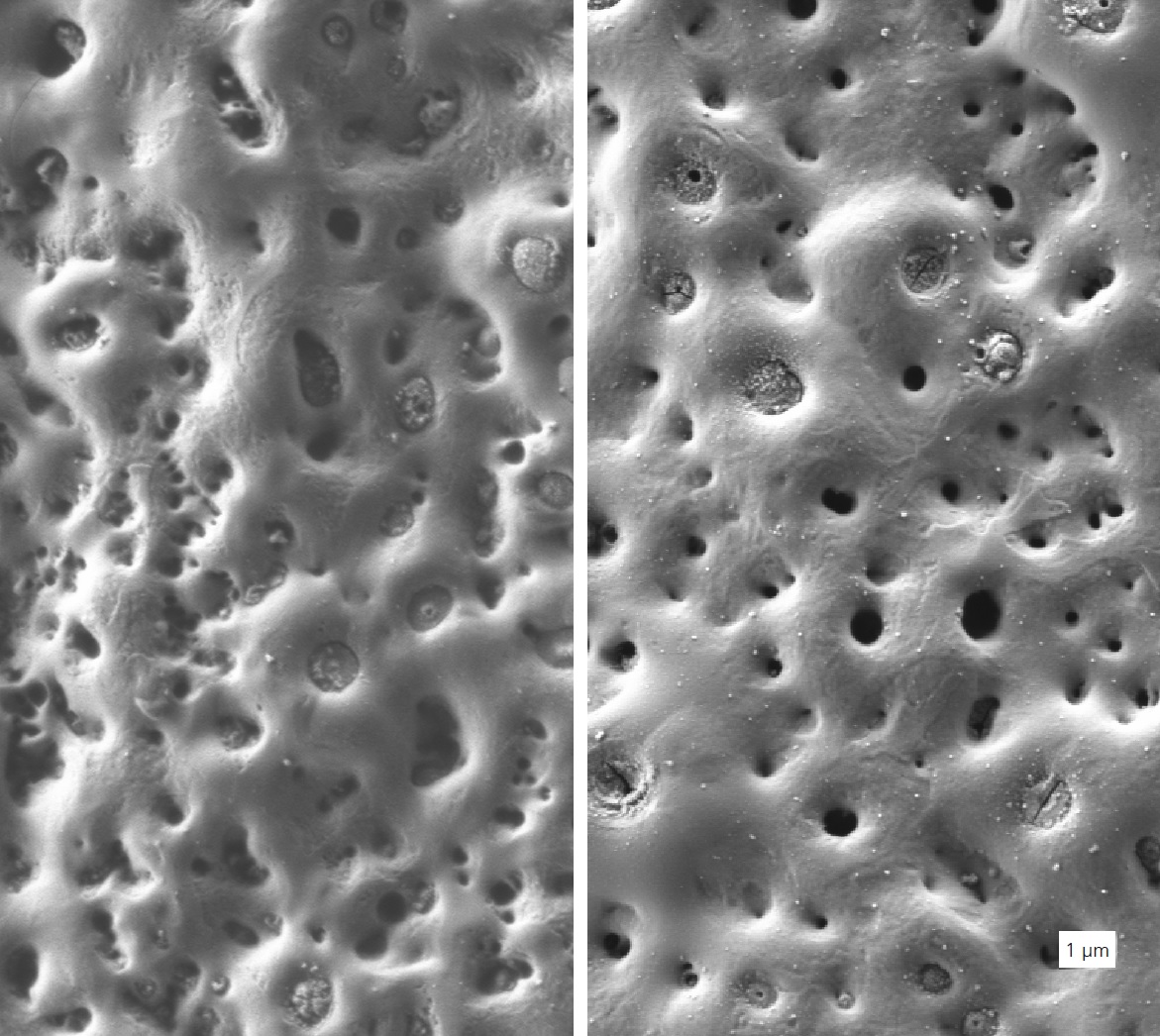
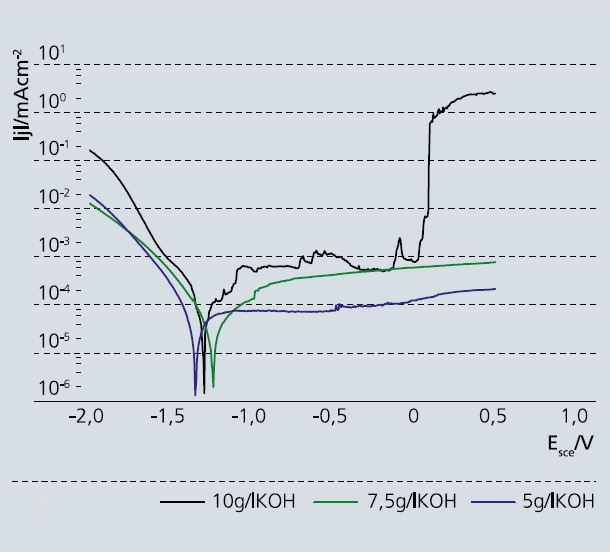
The high susceptibility of magnesium alloys to corrosion greatly restricts their use in lightweight construction. Previous attempts to improve the corrosion protection mainly involved organic coating systems on magnesium alloys. A further option is afforded by plasmaelectrolytic oxidation. This technique is similar to conventional anodizing in terms of the equipment used but employs a much higher applied voltage. This leads to dielectric breakdown of the conventionally formed oxide coatings due to the higher field strengths. The electric field strength causes ionization of the oxygen gas generated on the electrode interface, resulting in discharge of microscopic arcs (microsparks) on the surface of the material. The lifetime and the number of these sparks depend on the conditions, e.g., voltage or bath composition, under which the sparks are formed. This process is associated with pronounced localized heat generation, which causes the metals to be locally melted and thermochemically oxidized. The formed oxides are usually crystalline high-temperature modifications (e.g., MgO) with typical properties of oxides such as high resistance to chemicals. Due to the high resistivity, no electron transfer reactions (e.g., oxygen reduction) take place. Therefore, corrosion reactions are strongly inhibited. In the past, plasmaelectrolytic oxidation was usually carried out in fluoride-containing electrolytes. Recent research and development work has been focused on using fluoride-free electrolytes, which are less harmful in terms of health, safety, and the environment. One drawback of plasmaelectrolytic oxidation is the use of high voltages, which is associated with high energy consumption. Therefore, a further objective of research is to decrease the breakdown voltage. The oxide layers formed usually exhibit a number of pores or sinkholes, which can act as sites for initiation of localized corrosion.
For approximately two years now, the working group on electrochemistry at Fraunhofer IKTS has been collaborating with colleagues at DECHEMA-Forschungsinstitut to develop a novel procedure that works with a lower breakdown voltage and in fluoride-free anodizing electrolytes and that allows for the simultaneous incorporation of encapsulated corrosion inhibitors into the oxide layer. The aim is to achieve a significant improvement in the corrosion protection of plasmaelectrolytic oxide layers on magnesium alloys.
Funding of this work by AiF (grant no. IGF 472-ZBG) is gratefully acknowledged.