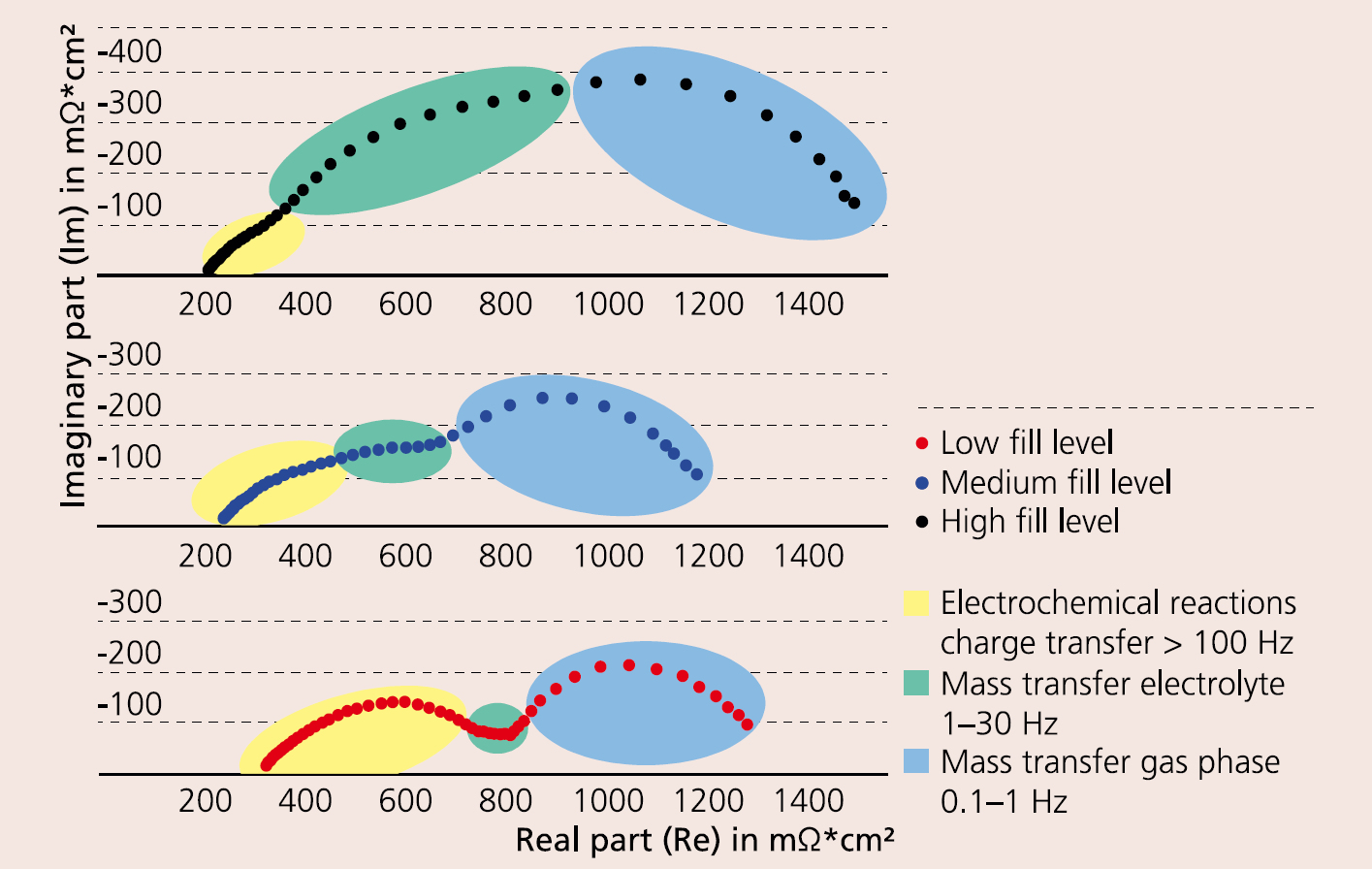
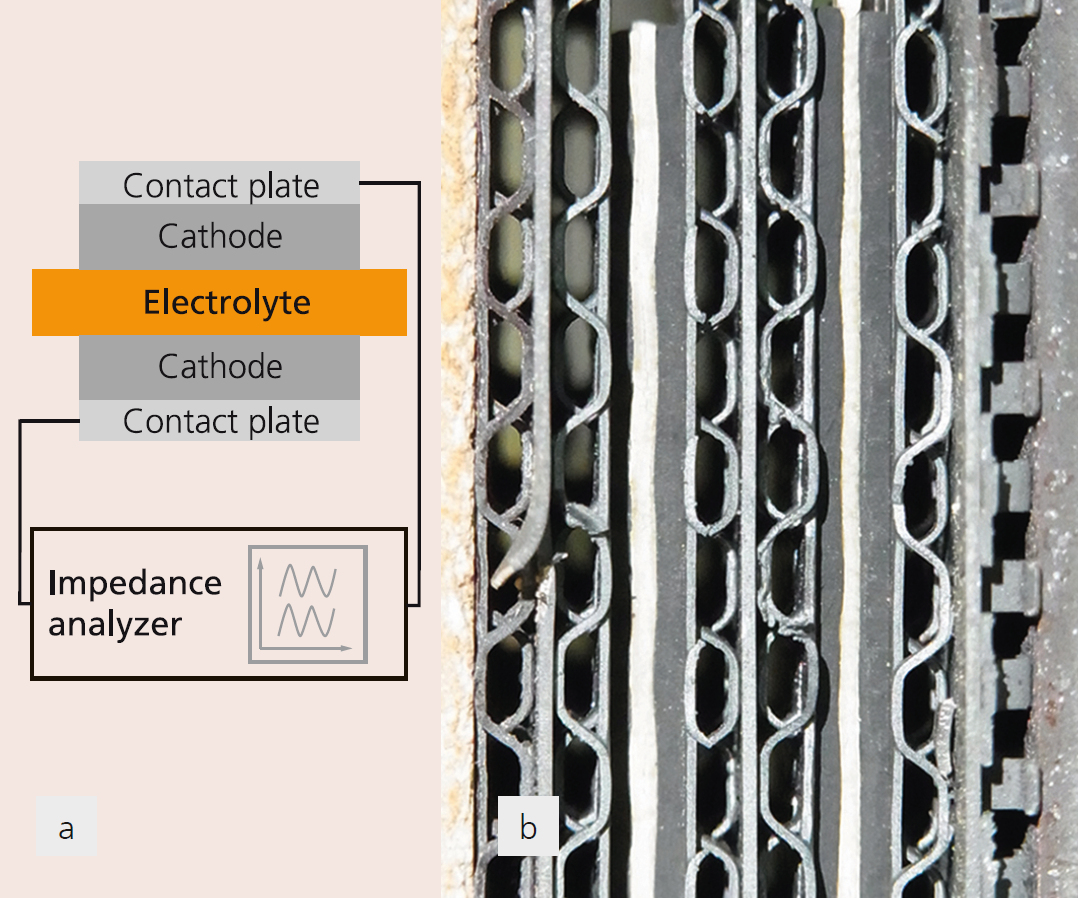
The molten carbonate fuel cell (MCFC) is at present one of the most mature and efficient fuel cell technologies in the field of stationary and decentralized power supply. Efficiency and service life are mainly determined by voltage losses at the electrochemically active electrodes. In comparison to the anode side reaction, the cathode side shows higher losses and has therefore a higher potential for optimization. The fundamental mechanism of the oxygen reduction in carbonate melts has been already extensively investigated. However in most cases, idealized experimental setups were used, which only insufficiently describe the processes at real porous cathode electrodes for short-term operation. The in-situ impedance characterization of cathodes under simulated operating conditions (600–700 °C; in O2/CO2/N2/H2O atmospheres) provides useful insight in the reaction mechanism and processes occurring at the cathode. Fraunhofer IKTS has carried out impedance measurements of cathode half cells (CHC) using the same cathode material for working and counter electrodes. An advantage of this method compared with the characterization of a full single cell is the prevention of superimposed processes of the anode side reaction. The measurement in a wide frequency range from 10 mHz to 100 kHz enables the separation and evaluation of electrochemical processes with differing time constants, such as charge transfer at interfaces, and diffusion processes (mass transfer). This allows for investigating the impact of single operating parameters, such as temperature, gas concentration and lifetime, on the reaction steps, enabling the targeted optimization of the cathode. Three different processes can be identified for MCFC cathodes: electrochemical reactions at interfaces, mass transfer inside the electrolyte and mass transfer inside the gas phase. In a Nyquist plot (Figure 1), individual electrochemical processes are represented schematically with half circles. Due to similar time constants of the single reaction processes, these half circles show a high degree of superposition at times. The investigations on cathodes with different fill levels of electrolyte inside the porous nickel-oxide structure have shown that the voltage loss by mass transfer can be decreased significantly by reducing the fill levels. However, such a reduction must be limited, since a high loss of electrolyte or super-low fill levels will increase electrode degradation. This in turn can lead to much higher voltage losses caused by the electrochemical reactions – which can be seen in the high frequency region of the impedance spectrum. The trials have shown that impedance spectroscopy at CHC is an excellent tool to characterize complex electrochemical systems like MCFCs and to precisely optimize them in terms of special parameters. The process is suitable for the characterization of electrochemical processes at high temperatures and diverse operation conditions and can be transferred to other fields, such as high-temperature batteries.