
Zeolite membranes for the energy-efficient separation of CO2 from biogas
Current research


Membrane technology is ideally suited for the energy-efficient removal of CO2 from biogas. Inorganic membranes are suitable candidates for CO2 separation because they have a high hydromechanochemical stability and good permeation properties. Zeolites, for example,have special properties, such as an intrinsic molecular sieving capacity, which means they can separate molecules of different sizes. Additionally, they are capable of preferential adsorption of certain gases. Chabasite (CHA) zeolites were chosen to fabricate membranesthat separate CO2 from biogas (CH4). Pore size in the CHA framework is similar to CH4 molecules but larger than CO2. In this research project, ultrahigh-flux CHA membranes with a high Si/Al ratio (SSZ-13) were produced for CO2/CH4 separation.
Asymmetric porous Al2O3 monotubular supports (10 mm outer diameter, 7 mm inner diameter, 200 nm average pore size in the separation layer, and a membrane surface area of ~17 cm2) were chosen for the zeolite membrane (top image).
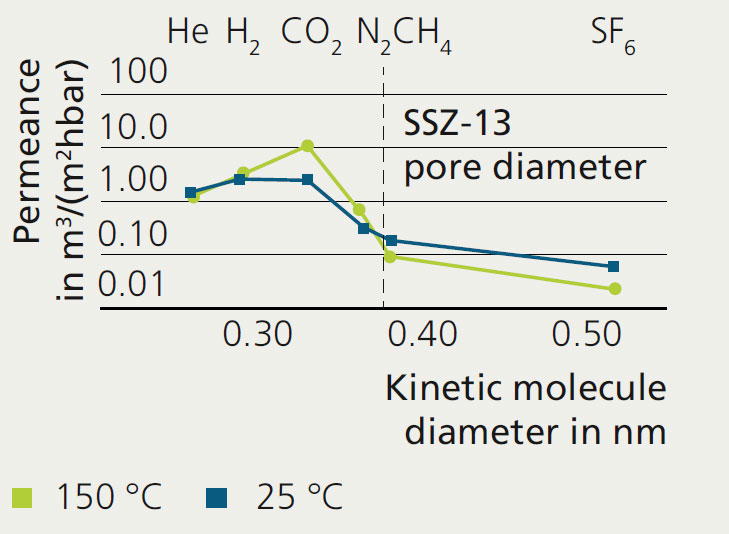
To evaluate the separation properties of the membrane, the permeation of single gases (middle figure) and the more complex permeation of binary gases (bottom figure) were analyzed. The single gas permeation of SSZ-13 membranes was tested at room temperature (here the dominant separation mechanism is adsorption) and at 150 °C (diffusion). In both cases, CO2 has the highest permeance of the tested gases. The ideal CO2/CH4 permselectivityreaches up to 112 with a CO2 permeance of around 11 m3/(m2hbar).
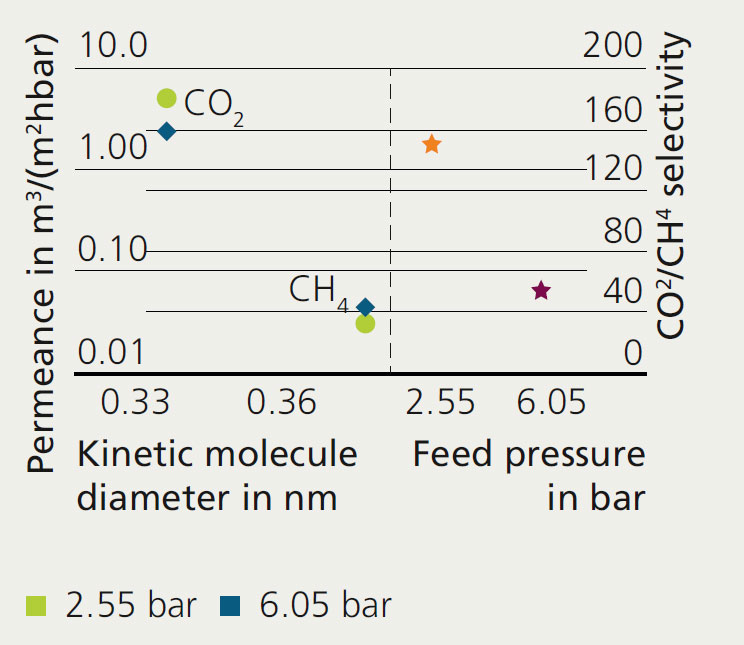
During the mixed gas permeation measurements, a gas mixture of 50 vol % CO2 and CH4 each was evaluated at room temperature with two different feed pressures. At the feed pressure of 2.55 bar, the CO2 permeance is high at ~5 m3/(m2hbar) and the permeance of CH4 is around 0.033 m3/(m2hbar). Accordingly, a CO2/CH4 selectivity of > 150 was achieved. An increase of feed pressure to 6.05 bar led to a decrease of the CO2 permeance down to ~2.5 m3/(m2hbar) and an increase of the permeance of CH4 to ~0.045 m3/(m2hbar). The CO2/CH4 selectivity of the SSZ-13 membranes decreased at higher pressure, but still reached a satisfactory value of ~57.
The results show that the novel zeolite membranes provide excellent separation performance – both in terms of CO2/CH4 selectivity and CO2 permeance. These membranes thus offer, for the first time, the possibility of separating highly concentrated CO2 from biogas in an energy-efficient manner in just a single membrane stage. The next step is now to upscale the membranes with partners from industry while maintaining the same separation performance.
We gratefully acknowledge the financial support of the European Union through the project “INNOMEM” (grant no. 862330–INNOMEM–H2O2–NMBP–TO–IND–2010–2020/H2O2–NMBP–HUBS–2019).
Sponsored by

