Biomaterial testing 2.0 – standardized, resource-efficient: ClicKit-Well
Current research



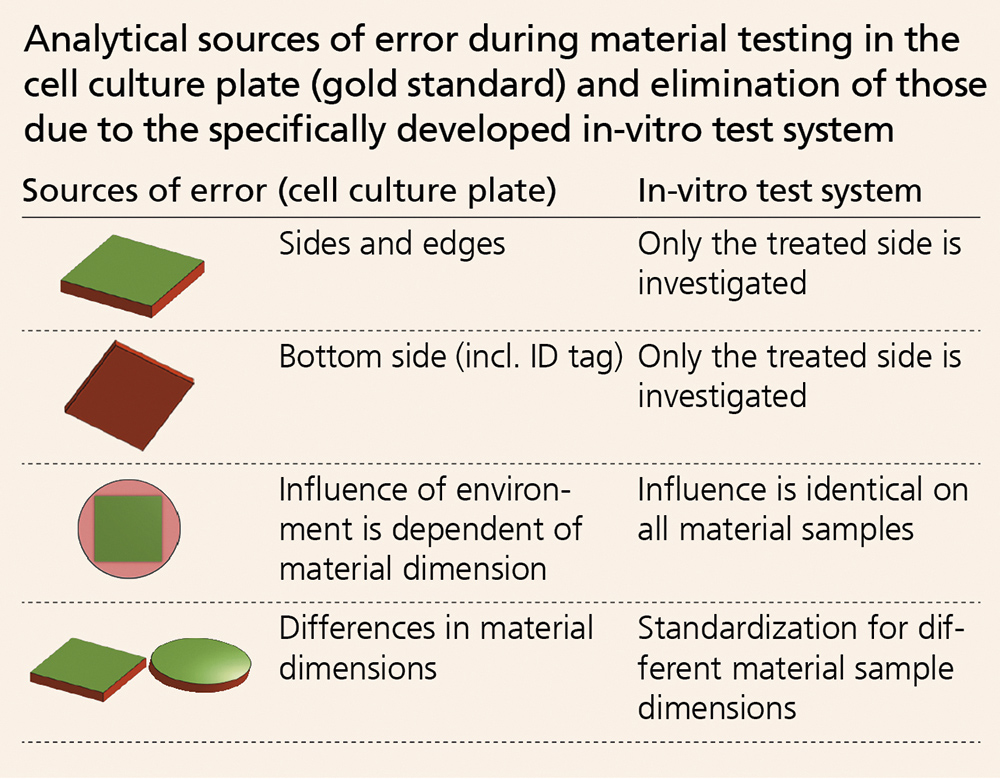
The rapid development of patient-specific innovative implant materials requires reliable, standardized test procedures and systems. These are vital for a preventative evaluation of material safety and functionality. Currently, biological tests are carried out in plastic cell culture plates (gold standard). In this method, samples are placed into an indentation (well). However, this leads to a number of sources for errors (see table).
Novel in-vitro test system (ClicKit-Well)
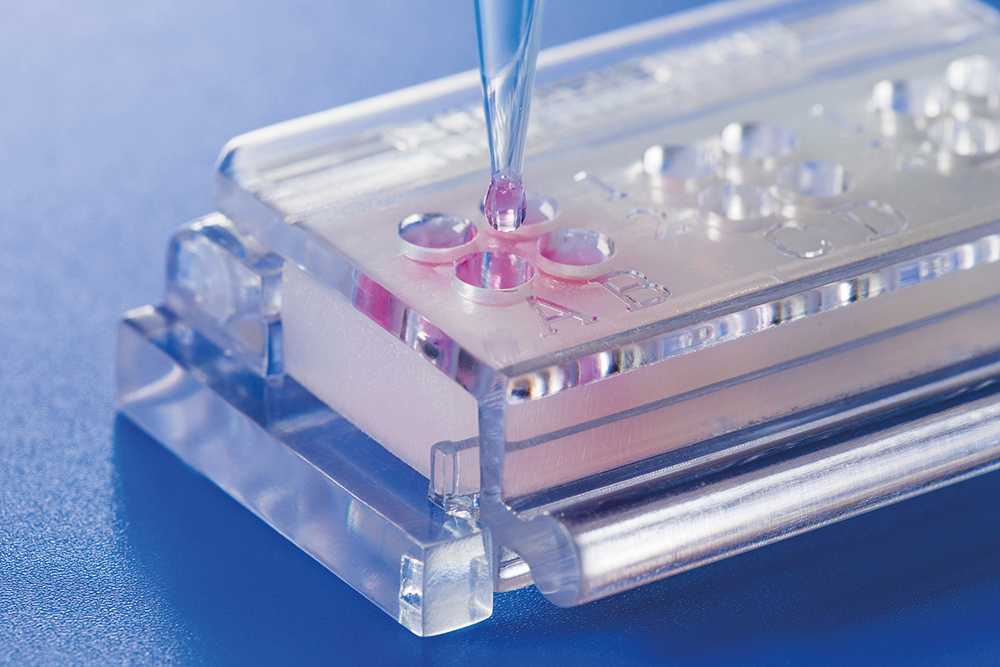
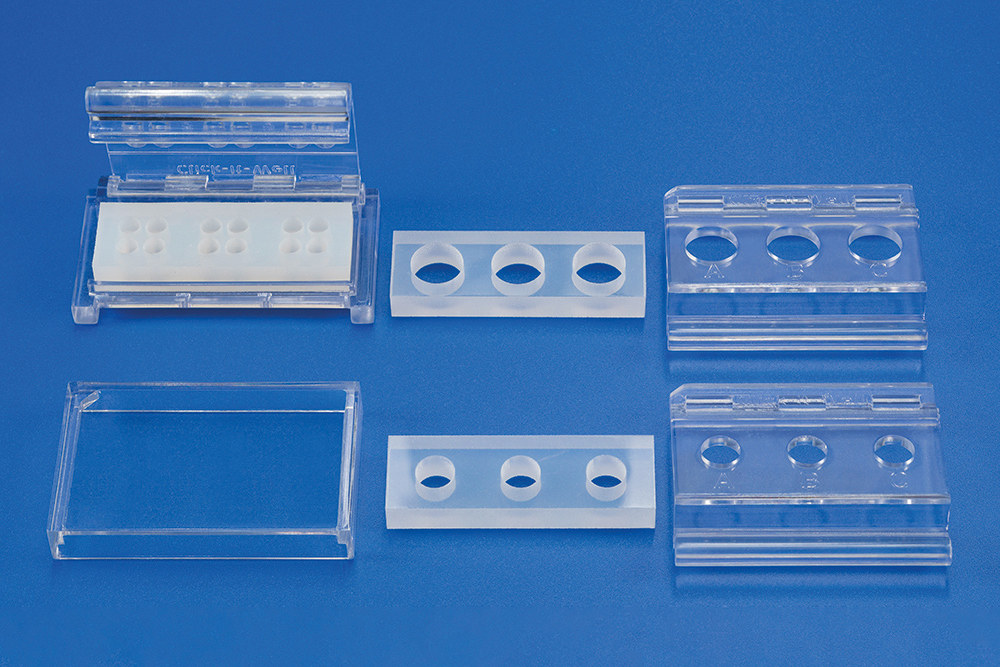
To reduce these errors, an in-vitro test system, “ClicKit-Well” (DE 10 2018 221 415.8, 12/18), was developed at Fraunhofer IKTS. The device creates standardized surface areas and wells (analog to common cell culture formats). This is achieved by attaching a deformable, perforated frame on top of the test material sample. Biological material can be introduced into the created wells and the interaction with the test material sample can be evaluated. The specific construction of the test system tolerates various surface characteristics (e.g. different roughnesses) and enables execution of multiple tests on a single material sample.
The in-vitro test system limits the contact of the biological material and the material sample to the surface that is to be tested. The specifically developed closing mechanism allows for a lasting, fluid-tight seal between frame and test material sample. The test system has a modular design and is constructed in the established 96-well, 48-well, and 24-well cell culture plate formats. The frame of the 96-well format version is designed so that up to four wells are generated at once on one test material sample. A covering lid that can be placed on the assembled test system makes sterile work possible.
The new approach – creating standardized surface areas on test material samples – provides a consistent test scenario that is more independent from material dimensions and minimizes the current sources of error of in-vitro testing.
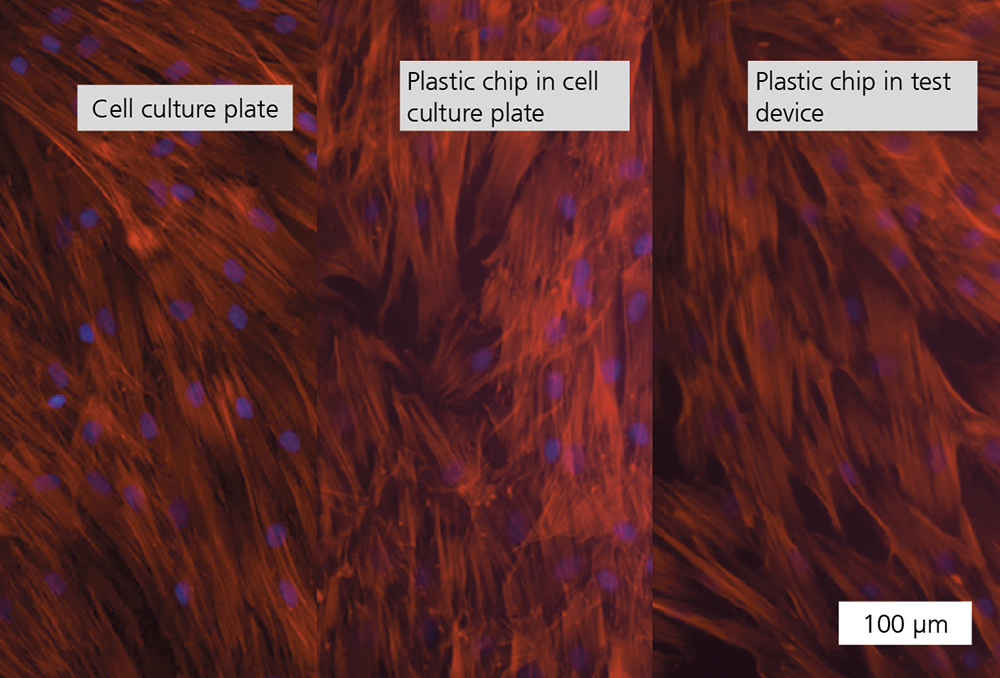
Feasibility study
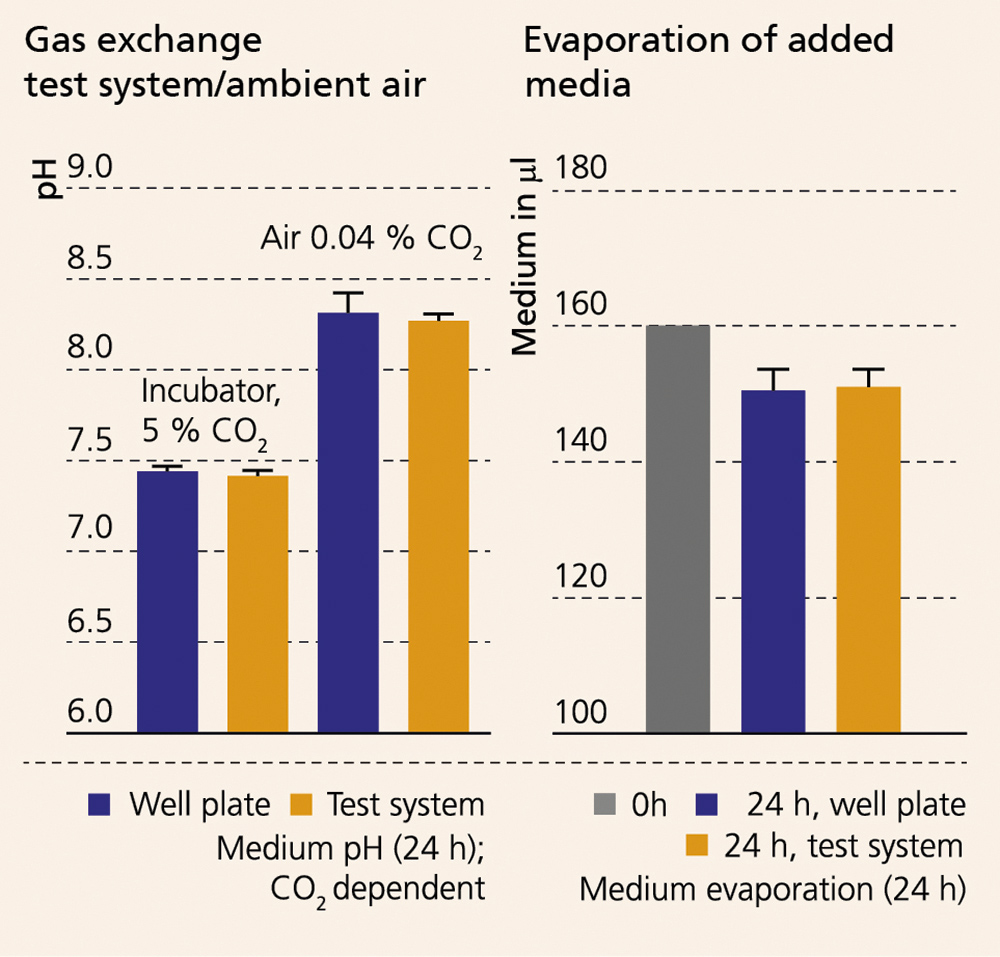
The gas exchange between test system and ambient air as well as the evaporation rate of added medium compared to the standard cell culture plate were tested in feasibility studies. To achieve ideal growth conditions of cultivated cells, it is necessary to ensure the gas exchange with the ambient air. Using a bicarbonate media buffer system, the ambient air in the incubator contains 5 % CO2 by default.
The proper gas exchange was tested by determining the pH value of the cell culture media after 24 hours of incubation in ambient air which contained 5 % CO2 and 0.04 % CO2 (Figure: Gas exchange). The incubation of the medium in ambient air containing 0.04 % CO2 resulted in the same increase in pH value for both the in-vitro test system and the cell culture plate. As expected, the media is buffered around an ideal pH value of 7.4 when incubated in the test system and the cell culture plate in ambient air containing 5 % CO2. Thus, the desired gas exchange with the ambient air and the test system could be demonstrated based on the dependence of the bicarbonate buffer system on the CO2 content in the surrounding ambient air. Similar results were shown for the analysis of the evaporation rate of the media over the course of a 24-hour incubation at incubator conditions (37 °C, humid atmosphere) (Figure: Evaporation). In a comparative cell culture experiment, human bone marrow stem cells (MSCs) were seeded into a 96-well cell culture plate and on plastic material samples that were placed in a cell culture plate as well as in the in-vitro test system at a density of 2.5 x 103/cm2/0.5 ml. Following a 24-hour incubation period, a nearly identical image of cell density and spread evolved in all three test scenarios.
The results show that the in-vitro test system is suitable for cell cultures. The next research phase will validate the qualification of the test system for broad use in the cell culture, e.g. for cell secretion and cell metabolism analysis. Technical requirements for market-relevant applications will be implemented and investigated.
Services offered
- Biological material assessment (safety and efficacy)
- Cell biological assays for direct application on material surfaces
- Miniaturization of biological in-vitro tests
- Development of standardized test scenarios
- Bio-interface studies: surface effects