Bio- and Medical Technology

Competences
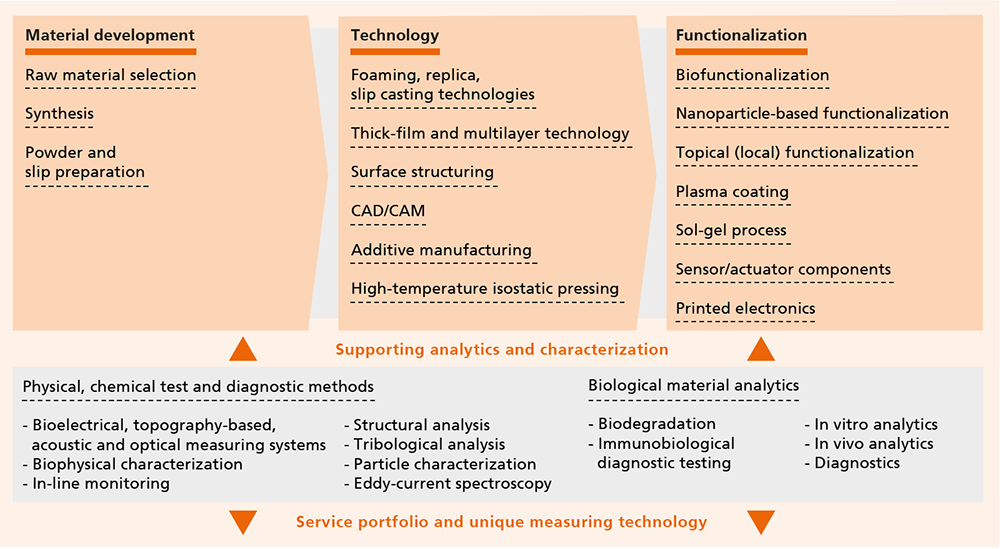
Ceramic materials and surfaces
- High-purity dense or porous bioceramics [Al2O3, ZrO2 (ATZ, ZTA, Y-TZP), Ca3(PO4)2), HAP, Si3N4]
- Open-cell foam ceramics and metal foams
- Glass and glass ceramics
- Oxide and non-oxide ceramics with precise electric, thermal, mechanical and optical functionalities
- Composites and material compounds (porous/dense, metal/ceramic)
Technologies
- Powder and slip preparation, granulate development
- Casting, pressing and (thermo)plastic shaping processes
- Foaming and replica technologies for cellular structures
- CAD/CAM line, additive manufacturing processes (powder- and suspension-based)
- Manufacturing of blanks and preforms by dry pressing
- Sample series of oxide ceramic semi-finished products (certified according to EN ISO 13485:2016)
- Plasma coating and sol-gel processes
- Thick-film and multilayer technology (complete line for HTCC, LTCC)
- Thin-film technology (thermal CVD, PECVD, thermal ALD, PVD, LPD)
- Joining technologies for ceramic/metal and ceramic/ceramic combinations
- High-temperature isostatic pressing/post-HIP processes
- Micro- and surface preparation; surface functionalization (inkjet and aerosol printing, diode laser array finishing)
- Biocompatible joining and packaging techniques, medical device construction
Diagnostic/therapeutic systems and characterization
- Bioelectrical, topography-based, acoustic and optical measuring systems
- Biophysical characterization on nano, micro and macro level (TEM, SEM, AFM, AFAM, Raman)
- In vivo as well as in vitro analysis and diagnostic systems
- Plasmonic sensor systems
- Biological evaluation of materials (medical products) in accordance with DIN EN ISO 10993
- Differentiation assays
- Development and industrial implementation of innovative in vitro test methods