The group ”Biological Material Analysis” develops preclinical, cell-based test models for the biological evaluation of medical products.
The development of biocompatible and immunocompatible medical products, such as medical devices and drugs, requires consideration of the interaction between materials or active substances and biology (biomolecules, cells, and tissues) to be able to use them safely on and in humans.
The group “Biological Material Analysis“ therefore pursues an interdisciplinary approach. At the interface of medical device or drug development and immunobiology, materials, substances and active agents are evaluated, as well as in cooperation optimized, or newly developed for industrial partners. Standardized diagnostic screening test procedures are also developed for this purpose.
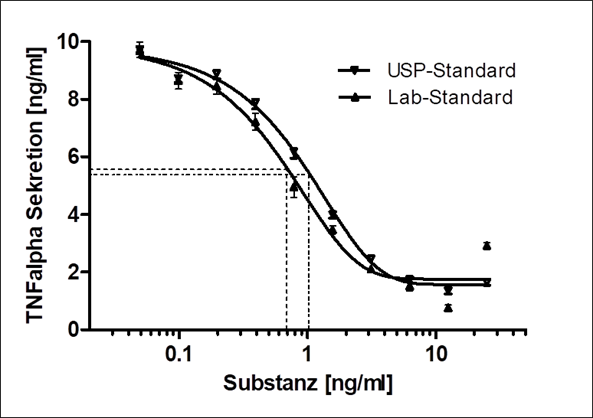
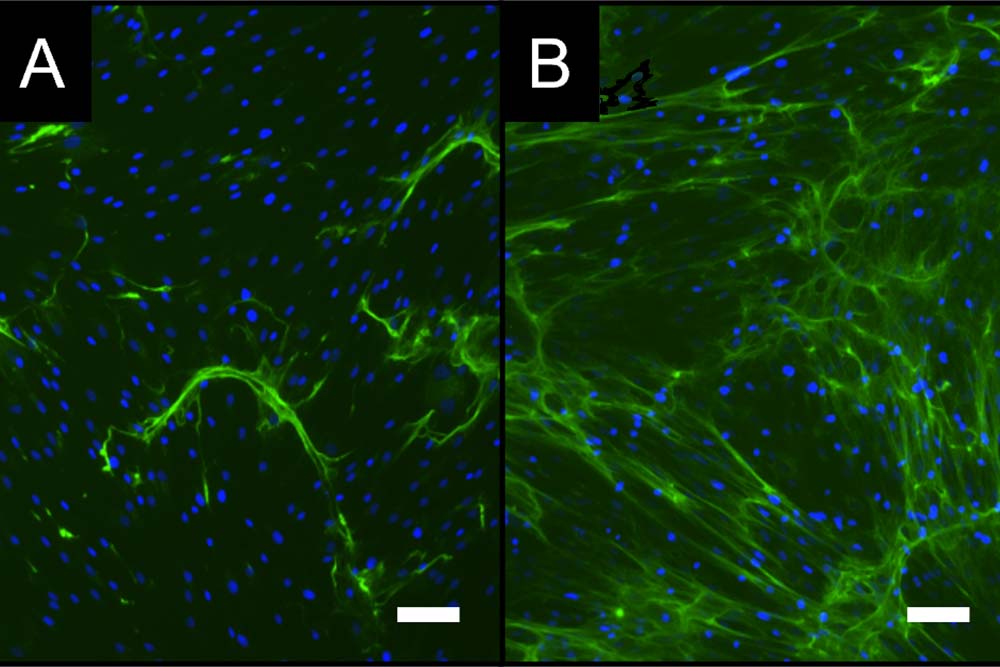
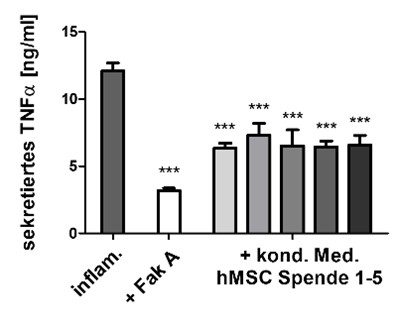
The focus is on cell-based test scenarios, test systems and analytical procedures for in vitro use. The developed processes form the basic framework for the questions to be investigated. In the implementation, the focus is primarily on physiological processes, i.e., processes in the patient. With such in vitro tests in the preclinical phase, the most suitable materials/active substances for the respective application can be identified and evaluated quickly and reliably, thus accelerating the development of novel medical products. The test scenarios are based on suitable acceptance criteria for method development and validation.
The Fraunhofer IKTS group’s place of work is at the Fraunhofer Institute for Cell Therapy and Immunology IZI in Leipzig. There are close cooperations with groups at the IZI with the aim to advance medical products and cell therapeutics for industrial applications.