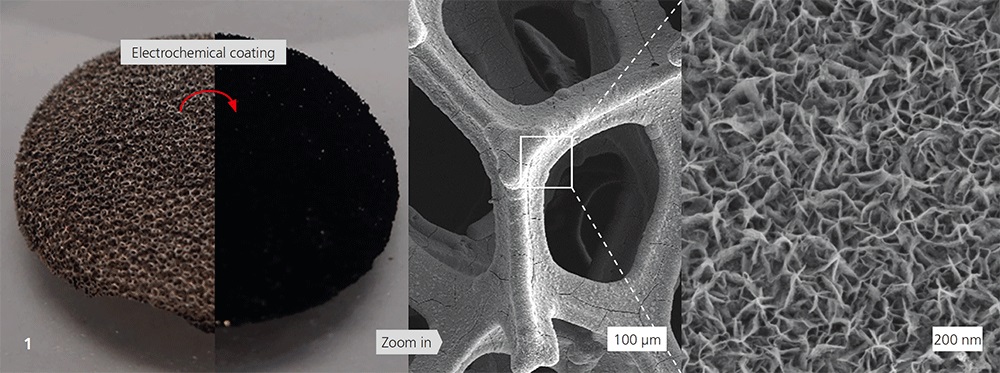
Oxygen gas diffusion electrodes (GDE) play a central role in alkaline energy converters, such as metal-air-batteries (Zn/O2) and polymer-membrane-electrolyzers (AEM-EL). GDEs provide the chemical reactions of oxygen evolution (OER) or oxygen reduction (ORR) but OER is the bottleneck for energy-efficient battery and electrolyzer technologies. The state-of-the-art GDE consists of carbon, electrochemical catalysts and PTFE (hydrophobic binder), of which carbon is particularly susceptible to corrosion. This shortens the service life of the GDE and thus also the operating time of the battery or electrolyzer. To counteract this, a carbon-free and therefore corrosion-resistant GDE was developed, which is bifunctional, meaning it is capable of both reactions (OER and ORR). Through the electrochemical deposition of manganese oxide on nickel foam (Figure 1), a carbon-free GDE was produced. Samples were prepared, characterized and compared with a commercial GDE (GDEref) in electrochemical measurements.
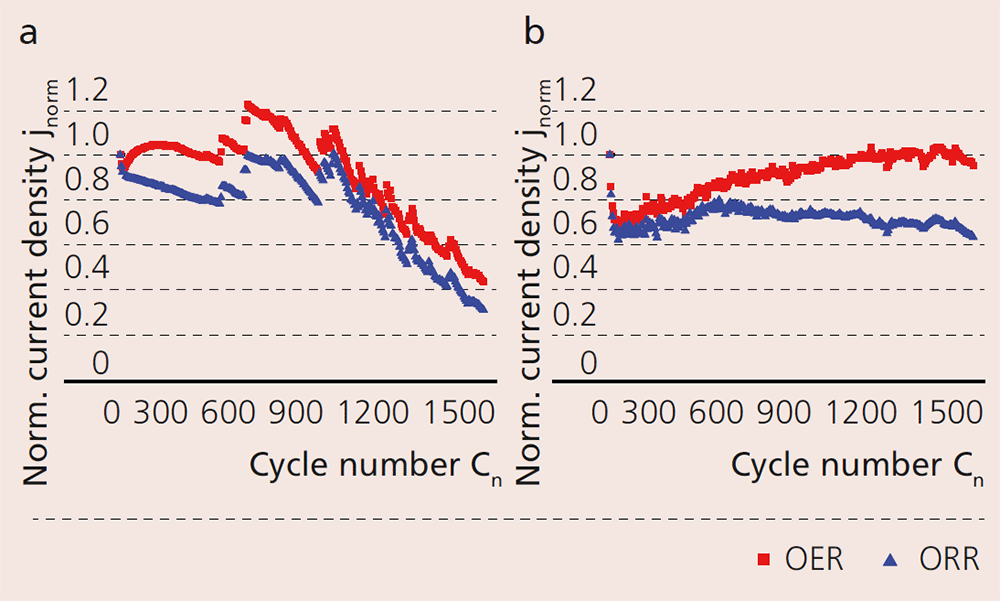
Oxygen evolution and oxygen reduction was measured for the commercial and the carbon-free GDE respectively, and the two were compared. A cycle represents a completed sequence of OER and ORR. The samples were run up to 1500 cycles and measurements for long-term stability (diagrams on the right) were performed. The results show a significant difference between the two types of GDE, with considerable advantages for the newly developed GDE.
The diagrams clearly show the carbon corrosion of GDEref (a). The performance reduction starts at approximately 900 cycles and continues steadily until it reaches around 60 % total loss. The carbon-free GDE however shows a mostly stable behavior for 1500 cycles. Following a drop of around 35 % in the first cycles, the capacity for OER (red) and ORR (blue) continually increases. Subsequently, ORR is maintained at the same level, while OER increases further until the last cycle.
The results presented here clearly demonstrate the potential of carbon- and precious-metal-free gas diffusion electrodes, which enable bifunctionality, higher current densities and voltages in alkaline metal-air batteries and polymer-membrane electrolyzers.
This work was funded by a scholarship from the Reiner Lemoine foundation.
