
Ceramic electrolytes for lithium and sodium solid-state batteries
Current research

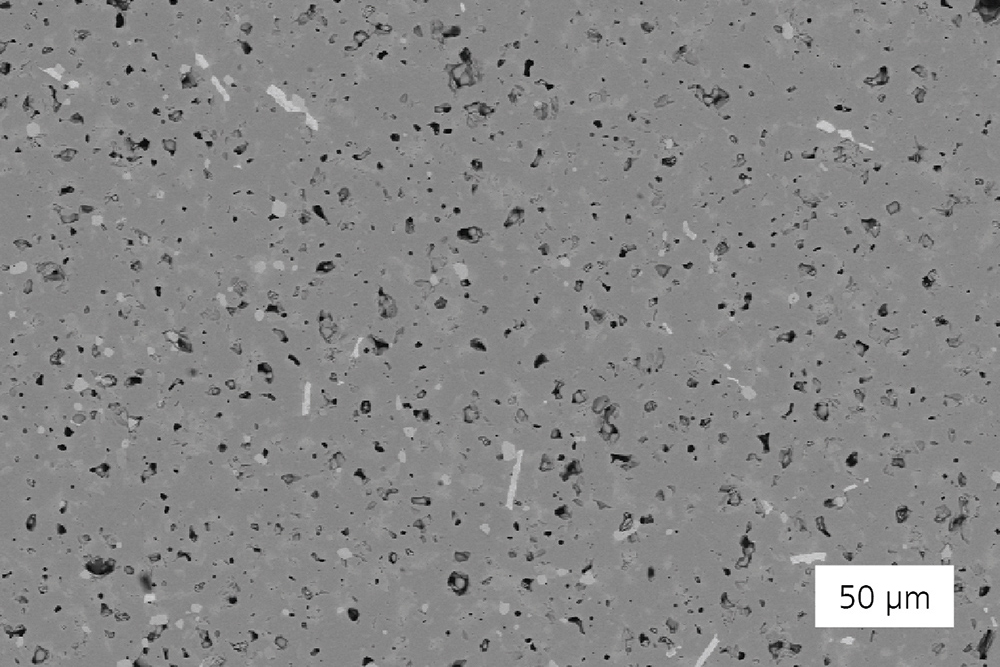
Motivation
Solid-state batteries (SSB) are considered a promising candidate for the next generation of batteries for automotive, industrial and stationary applications. The main advantages of this technology are improved safety thanks to the avoidance of flammable and harmful liquid electrolytes, and increased energy density thanks to the application of metal lithium or sodium anodes. Currently, very different material classes of solid electrolytes for use in solid-state batteries are being investigated and described. Polymer electrolytes have the advantage of high mechanical flexibility and compatibility with conventional manufacturing processes. However, their thermal stability and conductivity at room temperature are limited. In contrast, ceramic materials have many beneficial properties, which form the basis for new battery concepts.
Ambient-temperature secondary lithium SSB
With regard to room-temperature lithium batteries, one focus of the R&D activities at IKTS is on ceramic electrolytes based on oxide and phosphate materials (LLZO, LATP), which have a high electrochemical and chemical stability and ionic conductivity in the range of 10-3 to 10-4 S/cm. However, ceramics are brittle, potentially placing limits on its handling and lifetime. A particular challenge of this material class is the high sintering temperature required for densification, as well as the chemical interaction with the individual components of the composite electrode (active material, ionically conductive and electronically conductive phase) during co-firing. To resolve this challenge, the researchers looked at various approaches to reduce the sintering temperature of solid electrolytes. At Fraunhofer IKTS, NASICON-based structures, such as LATP (Li1+xTi2−xAlx (PO4)3) are investigated to develop strategies that achieve a reduction of sintering temperatures from 1100 °C to around 800 °C by using precursors as starting materials. However, chemical interactions of the active materials were observed during cofiring, which need to be avoided. These interactions are evident between NMC (Li(Ni,Mn,Co)O2) and LATP at well below 800 °C. Further approaches are in development to resolve this challenge. In addition, sulfide electrolytes showing an exceptionally high ionic conductivity but limited electrochemical and chemical stability are utilized for electrochemical cell manufacturing and tests. Current research efforts at Fraunhofer IKTS focus on optimizing boundary layers in order to overcome these limitations. Sulfides can also be processed with typical ceramic technologies, such as tape casting, but do not require a sintering step. With this regard, processes for coating active materials are of special interest.

Ambient-temperature secondary sodium SSB
Low-temperature sodium batteries have so far been in the shadow of Lithium battery development, as they have lower energy densities than common lithium batteries. Current trends in the development of solid-state batteries suggest the advantages of sodium-based batteries over lithium chemistry. Numerous active materials have already been developed which show energy densities comparable to those of lithium chemistry but are based on readily available and environmentally friendly raw materials. The ionic conductivity of inorganic solid electrolytes for sodium is similar to that of established materials for lithium, but processability with other components of solid-state batteries is much easier. Moreover, the integration of metal sodium as anode can boost energy density. A special glassceramic material group based on Na2O-Y2O3-P2O5-SiO2 (NaYPSiO), developed at IKTS, shows excellent processability using ceramic shaping technologies and high ionic conductivity (5 mS/cm) at 25 °C. Tests with sodium anodes on NaYPSiO electrolytes have shown low polarization resistance values. The use of this high-performance material in the development of battery components for solid electrolytes and active materials on the cathode side is the next step in realizing sodium-based systems for solid-state batteries.
High-temperature sodium SSB
While the previously mentioned battery types are used in the field of E-mobility, sodium-nickel chloride (or ZEBRA) batteries find commercial use in stationary energy storage solutions. They are low-cost since only nickel and rock salt are applied as active materials. Furthermore, their energy density is similar to that of lithium batteries (125 Wh/kg) and they typically operate at 300 °C. Fraunhofer IKTS is actively developing the entire chain of battery technology: from components, such as solid electrolytes and cathode materials, to battery cells in different designs (tubular, flat), to battery modules with integrated temperature management. The material beta-alumina has one of the highest ion conductivity levels found in solid electrolytes (~300 mS/cm at 300 °C and ~5 mS/cm at room temperature). It can generally also be used in room-temperature secondary sodium solid-state batteries. Beta-alumina is classically applied in tubular sodium-nickel-chloride batteries and produced by isostatic pressing. Fraunhofer IKTS has established several techniques for the straightforward shaping of betalumina, including tape casting, extrusion, uniaxial pressing and slip casting.
Conclusion
Solid-state batteries are an essential contribution to the future development of a sustainable energy economy. Ceramic materials and technologies are the focus of extensive battery research activities at Fraunhofer IKTS, because they can contribute to solving key technological challenges.
Download
Supported by
