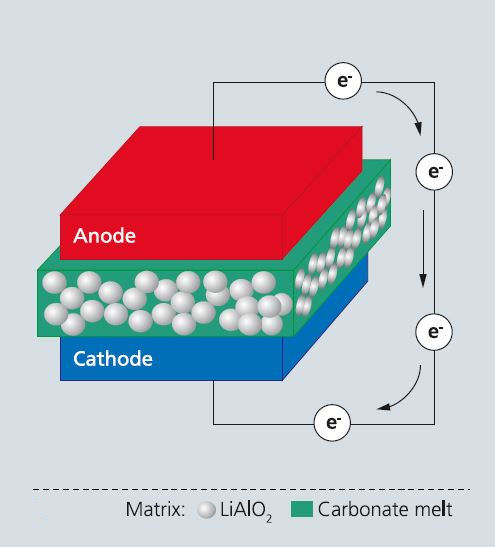

The Molten Carbonate Fuel Cell (MCFC) is presently one of the most mature and efficient fuel cell technologies, enabling electrical energy efficiencies of up to 48 % for 350-kW to 3-MW systems. Both the electrolyte storage capacity and the cell lifetime depend on the stability of the key component, the porous matrix made of submicron LiAlO2 particles holding the molten carbonate electrolyte by means of capillary forces. Particle coarsening and/or phase transformations during long-term operation may reduce the matrix electrolyte retention capability and impact cell life.
Although LiAlO2 nanopowder synthesis has already been reported, the stability in MCFC operating conditions remains an important challenge. The newly established Fraunhofer Attract group “Materials MCFC” works on addressing basic aspects of the effects of synthesis parameters on the coarsening behavior and phase transformations in LiAlO2 to enable more stable and simultaneously cost-efficient material. The research infrastructure covering powder synthesis, matrix preparation by tape casting, materials testing, and materials characterization in cells and half cells was established for this purpose. The solid- state reaction between AlOOH (Sasol Germany GmbH) and Li2CO3 (Sigma-Aldrich Chemie GmbH) was selected as the most promising approach due to its relative simplicity, good scalability, and cost-efficient starting materials. Different mixing/milling and drying approaches using either solvent- or water-based media were tested. Variation of calcination times and temperatures over wide ranges yielded information on the kinetics of the calcination process. The powders were characterized in terms of crystalline structure, porosity, crystallographic phases, and morphology using XRD, Brunauer-Emmett-Teller surface analysis, differential thermal analysis, and scanning electron microscopy. In the present work, the feasibility of chemically modifying the initial slurry in order to block undesirable LiAlO2 phase transformations during calcination at temperatures as high as 700 °C was demonstrated. This improvement resulted in a stable LiAlO2 phase over a wider temperature range and is important for high-temperature application of this material.